- 1Department of Cancer Epidemiology, Affiliated Cancer Hospital of Zhengzhou University, Henan Cancer Hospital, Henan Engineering Research Center of Cancer Prevention and Control, Henan International Joint Laboratory of Cancer Prevention, Zhengzhou, China
- 2Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Beijing Office for Cancer Prevention and Control, Peking University Cancer Hospital and Institute, Beijing, China
- 3The Clinical Epidemiology of Research Center, Department of Dermatological, The First Affiliated Hospital of Baotou Medical College, Baotou, China
- 4Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 5Department of Cancer Prevention, The Cancer Hospital of the University of Chinese Academy of Sciences, Zhejiang Cancer Hospital, Hangzhou, China
- 6Liaoning Office for Cancer Control and Research, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, China
- 7School of Nursing, Jining Medical University, Jining, China
- 8Department of Preventive Health, Xinxiang Central Hospital, Xinxiang, China
- 9Public Health School, Dalian Medical University, Dalian, China
- 10Department of Gastroenterology, Wuzhou Red Cross Hospital, Wuzhou, China
- 11Department of Cancer Prevention and Control Office, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- 12Department of Clinical Research, The First Affiliated Hospital, Jinan University, Guangzhou, China
- 13State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China
- 14Department of Student Affairs, Affiliated Tumor Hospital, Xinjiang Medical University, Ürümqi, Xinjiang, China
- 15Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing, China
- 16School of Public Health and Management, Chongqing Medical University, Chongqing, China
- 17Department of Public Health, Gansu Provincial Cancer Hospital, Lanzhou, China
- 18School of Public Health, Chengdu Medical College, Chengdu, China
- 19Center for Global Health, School of Population Medicine and Public Health, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Objectives: To explore the utilization, barriers, and factors associated with the targeted treatment of Chinese metastatic colorectal cancer (mCRC) patients.
Methods: A total of 1,688 mCRC patients from 19 hospitals in 14 cities were enrolled from March 2020 to March 2021 using stratified, multistage cluster sampling. The use of targeted therapy and any barriers patients experienced were collected. Logistic regression analyses were conducted to identify the factors associated with initiating targeted treatment.
Results: About 51.6% of the patients initiated targeted therapy, of whom 44.5%, 20.2%, and 35.2% started first-, second-, and third-line treatment, respectively. The most reported barriers were high medical costs and a lack of belief in the efficacy of targeted therapy. Patients treated in the general hospital, diagnosed at an older age, less educated, and who had a lower family income, no medical insurance, poor health-related quality of life, metastasis outside the liver/lung or systemic metastasis, a shorter duration of mCRC were less likely to initiate targeted therapy.
Conclusion: Reduced medical costs and interventional education to improve public awareness could facilitate the use of targeted treatment for mCRC.
Introduction
Colorectal cancer (CRC) is the most common type of gastrointestinal cancer worldwide and the third leading cause of cancer-related death, resulting in an estimated 1.9 million new cases and about 935,000 deaths each year [1]. The burden of CRC varies greatly by geographic region, likely due to differences in lifestyle and socio-economic status [2]. China has the largest number of cases and CRC-related deaths as a result of its large population [3]. In 2016, it was estimated that about 408,000 new CRC cases and 195,600 CRC deaths occurred in China, giving it the second highest incidence and fourth highest mortality rate of all cancers [4]. While early screening and treatment have reduced the number of deaths [5], 25% of CRC patients are diagnosed with metastases, and 50% will go on to develop metastases [6], resulting in poor prognosis and a heavy disease burden.
Chemotherapy is the primary treatment for metastatic CRC (mCRC) [7]. However, chemotherapy alone is often limited by the lack of tumor cell selectivity, insufficient drug concentration in tumor tissues, systemic toxicity, and the appearance of drug-resistant tumor cells [8, 9]. According to the Pan-Asian adapted ESMO consensus guidelines for the management of patients with mCRC, the chemotherapy should be combinations of cytotoxic agents, in singlet, doublet, or combination chemotherapy along with appropriate targeted biological agents [10]. Over more than 10 years, the remission and survival conditions of mCRC patients have been revamped mainly because of development in prescribing conventional chemotherapy in combination with targeted agents. For example, clinical data indicated that the addition of bevacizumab to chemotherapy using fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) prolonged median progression-free survival (PFS) from 9.7 to 12.1 months, and increased the overall response rate from 53% to 65% among the untreated mCRC patients [11]. A randomized controlled trial among Chinese patients with RAS wild-type mCRC found that first-line cetuximab combined with leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) improved the PFS (9.2 vs. 7.4 months), overall survival (OS) time (20.7 vs. 17.8 months) and overall response rate (61.1% vs. 39.5%) compared with FOLFOX-4 alone [12]. A meta-analysis demonstrated that aflibercept plus leucovorin calcium, fluorouracil, and irinotecan hydrochloride (FOLFIRI) showed better survival efficacies as a second-line treatment for mCRC compared with FOLFIRI alone [13]. In mCRC patients who had progressed following treatment with all approved standard drugs, both regorafenib and fruquintinib treatment offered substantial survival benefits. Compared with the placebo, patients treated with regorafenib or fruquintinib had prolonged OS and PFS [14–17].
Given the recognized clinical benefits of targeted cancer therapy, many countries and professional organizations have approved its use in clinical practice [18, 19]. In China, four targeted agents (bevacizumab, cetuximab, regorafenib, and fruquintinib) for mCRC were approved and listed in the medical insurance directory [19]. Bevacizumab, a recombinant humanized immunoglobulin monoclonal antibody that binds to and inhibits the activity of human vascular endothelial growth factor-A (VEGF-A), was approved for mCRC treatment in 2010 and is now used as a first- or second-line therapy in combination with chemotherapy [20, 21]. Cetuximab, an anti-epidermal growth factor receptor (EGFR) monoclonal antibody, was approved in China in December 2005 as a second- or third-line medication for mCRC and as a first-line therapy in combination with chemotherapy in September 2019 [12, 22]. Regorafenib, an oral multi-kinase inhibitor that blocks and inhibits the activity of multiple protein kinases including vascular endothelial growth factor receptors (VEGFRs), fibroblast growth factor receptor (FGFR), platelet-derived growth factor (PDGF)-α and -β, epidermal growth factor (EGF), angiotensin 2, and the Rapidly Accelerated Fibrosarcoma kinase pathway, was approved as the third-line therapy for mCRC in March 2017 [19, 23]. Fruquintinib, which inhibits VEGFR signaling was approved as the third-line therapy for mCRC in September 2018 [19, 24]. However, the use of targeted agents in real-world clinical practice has never been investigated. Thus, this study sought to explore the use of targeted therapy, identify potential barriers to its utilization, and identify factors associated with treatment initiation by Chinese mCRC patients. The goal was to provide reference data for clinicians and policymakers to prolong mCRC patient survival.
Methods
Study Design
This is a multicenter, cross-sectional, hospital-based clinical epidemiology study conducted in China, using stratified, multistage cluster sampling.
Selection of Hospitals
The survey methodology was previously described [25]. In brief, China was stratified into seven geographic regions (East, North, South, Central, Northeast, Southwest, and Northwest) according to the traditional administrative district definition. The CRC disease burden differs by region [3]. During stage one, two cities from each geographical region were selected by simple random sampling. During stage two, one tertiary cancer hospital and/or one general hospital that represents the regional resources available to patients in each city were selected through convenience sampling based on their ability to provide CRC diagnosis, surgery, radiotherapy, chemotherapy, and routine follow-up care. A total of 19 tertiary hospitals (10 oncology and nine general hospitals) in 14 cities were involved in the study (Supplementary Figure S1).
Selection of Patients
All mCRC patients at the selected hospitals were included if they were ≥18 years of age, had metastases and finished at least one cycle of chemotherapy, and provided informed consent. Patients were excluded if they had severe physical, cognitive, and/or verbal impairments that interfered with their ability to complete the questionnaire, or if their medical records on the use of targeted agents were unavailable.
Data Collection
Well-trained clinical interviewers administered the survey to collect information on patient demographics, social structure, clinical results, utilization of targeted therapy, and barriers to treatment. The interviewers were clinicians or nurses from the selected hospitals, and members of the China Working Group on CRC Survey. Andersen’s behavior model for healthcare utilization was the framework used to identify the predisposing, enabling, and need-for-care factors affecting patient initiation of targeted therapy [26, 27]. Factors included in the model were self-reported perceived barriers and patient characteristics associated with targeted treatment (Figure 1).
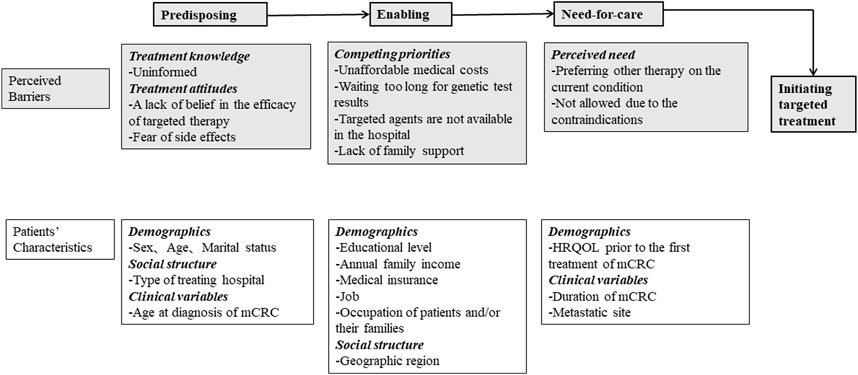
FIGURE 1. Framework of predisposing, enabling, and need factors for initiating targeted treatment. HRQOL, health-related quality of life; mCRC, metastatic colorectal cancer (China, 2023).
Study Measures
Utilization of Targeted Therapy and Self-Reported Barriers
Patient use of targeted treatment was collected through medical records. If patients had ever used a targeted agent, they were defined as “initiating targeted therapy,” otherwise, they were defined as “not initiating targeted therapy.” Of the patients who were “initiating targeted therapy,” the timing (first-, second-, and third-line) and types of targeted agents first used were obtained from medical records. Of those who were “not initiating targeted therapy,” related reasons or barriers to treatment were obtained through additional interviews administered by the clinical interviewers.
Patient Characteristics
Patient demographics, social structure, and clinical characteristics were collected from medical records and/or a standardized self-report questionnaire. The independent variables potentially associated with the initiation of targeted agents were selected using Andersen’s behavior model.
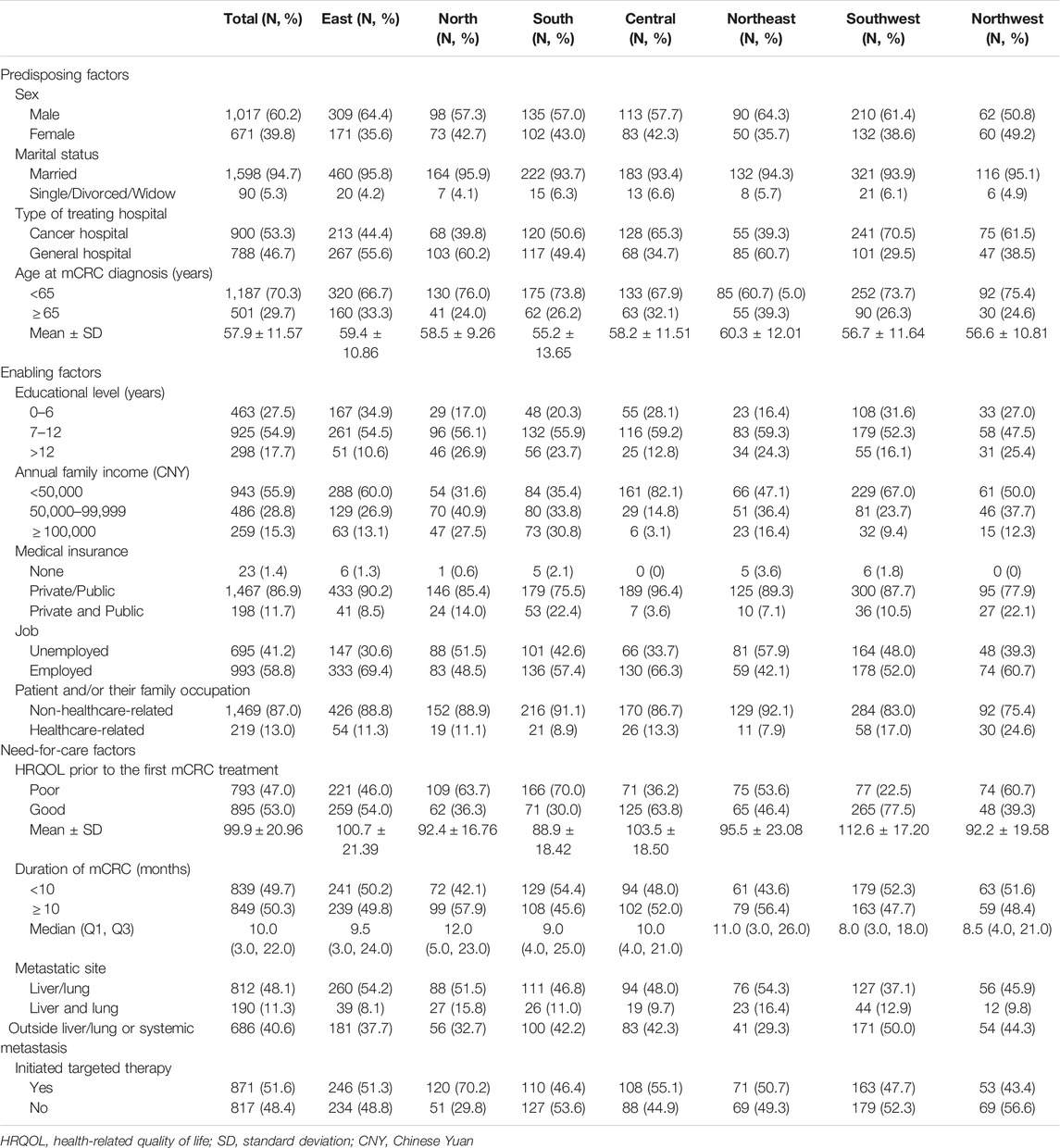
i. Predisposing factors included sex, marital status (married, single/divorced/widowed), and type of treating hospital (cancer, general), and age at diagnosis of mCRC (<65, ≥65 years of age).
ii. Enabling factors included educational level (0–6, 7–12, >12 years), annual family income (<50,000 CNY, 50,000–99,999 CNY,
iii. Need-for-care factors included health-related quality of life (HRQOL) prior to the first mCRC treatment, duration of mCRC (<10 and ≥10 months), and metastatic site (liver/lung, both liver and lung, outside liver/lung or systemic metastasis). HRQOL score was determined using the traditional Chinese Functional Assessment of Cancer Therapy-Colorectal (FACT-C, Version 4). It covers five function subscales (physical, social/family, emotional, functional, and colorectal cancer). Each item was ranked using a 5-point Likert scale (0–4). The total scores were calculated (ranging from 0 to 136) and classified as “poor” (total score ≤100) or “good” (total score >100) [28].
Data Management and Quality Control
A non-identifiable paper-based questionnaire was used to manage individual participant data. Data obtained from the questionnaire were consistent with the participant self-report and medical record information. All collected data were double entered using Epidata software V.3.1 by two trained research assistants and an independent data administrator compared the entries to flag any discrepancies between the two datasets. The data were then checked using SAS software V.9.4 and any data queries were sent to the investigators to be resolved.
Statistical Analysis
Descriptive analyses were used to report patient characteristics, distribution of the treatment timing (first-, second-, and third-line), the kinds of targeted agents used, and any identified barriers to treatment. Categorical variables were described as absolute frequencies and percentages, normally distributed continuous variables were shown using the mean and standard deviation (SD), and abnormally distributed continuous variables were described using the median and standard interquartile range (IQR, Q1–Q3).
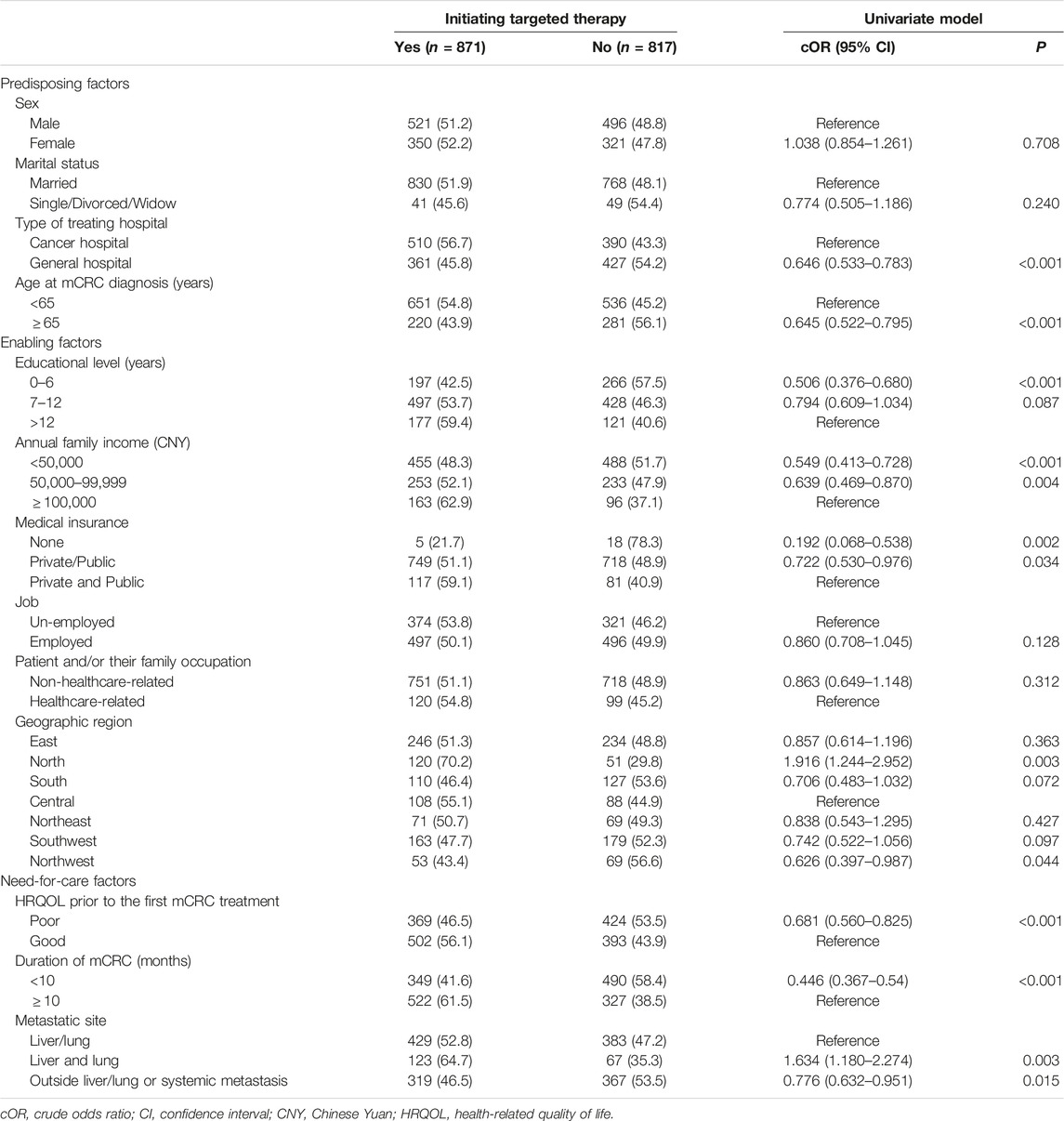
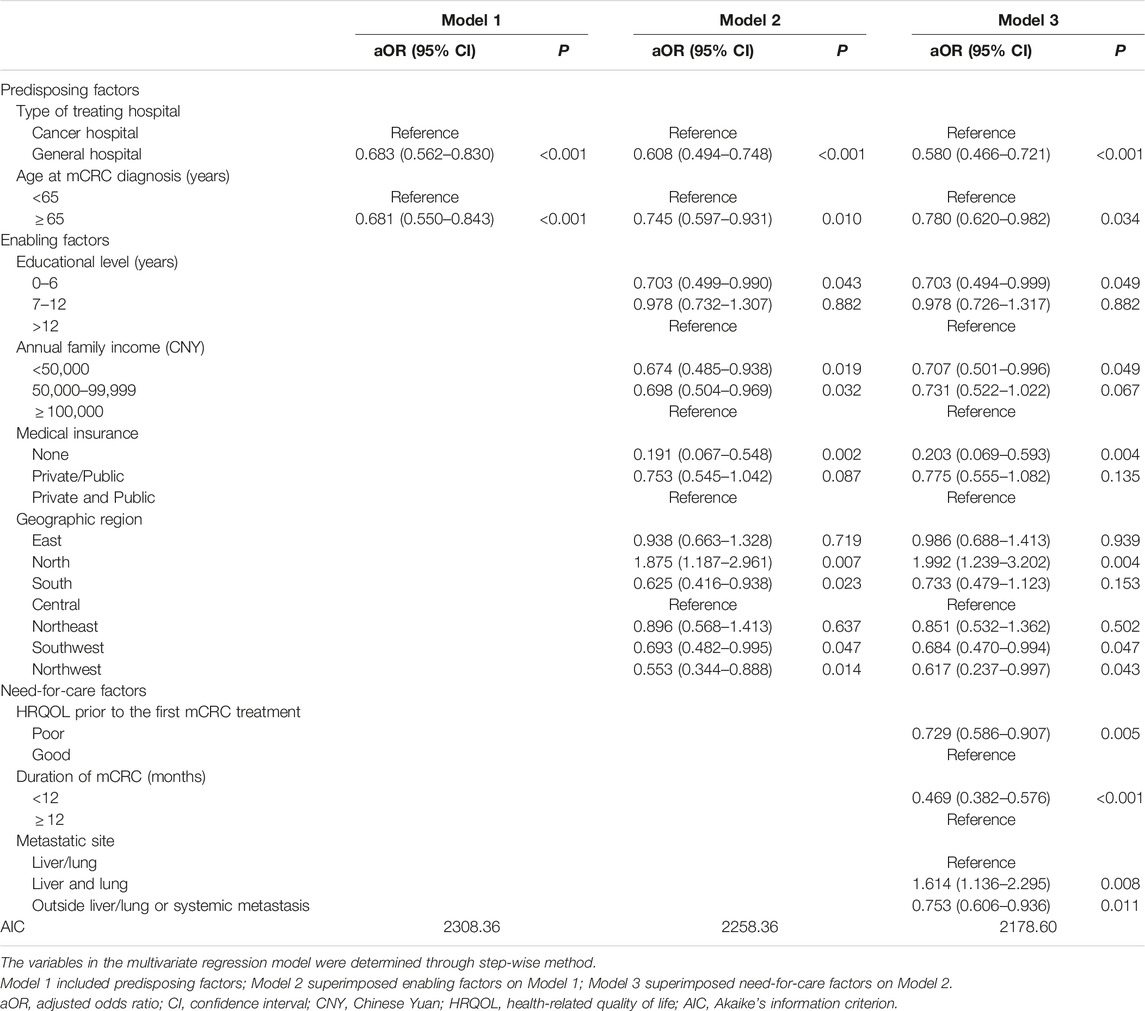
Univariate and multivariate logistic regressions were conducted to identify the factors that correlated with initiating targeted therapy. Variables with a p-value < 0.1 in the univariate logistic regression model were candidates for multivariate regression analysis. A sequential series of multivariate regression models were developed to create the final multivariable model. Model 1 included predisposing factors, Model 2 superimposed enabling factors on Model 1, and Model 3 superimposed need-for-care factors on Model 2. The variables in the multivariate regression model were determined with the stepwise use of Akaike’s information criterion (AIC), and the model with the minimum AIC was adopted as the final model. Crude odds ratios (cOR) with 95% confidence intervals (CI) were reported for the univariate model, and adjusted odds ratio (aOR) with 95% CI were reported for the multivariate model. Sensitivity analysis was also conducted by limiting to patients with a mCRC duration of ≥10 months, to control for the impact of short-time duration on the use of targeted therapy. All statistical analyses were conducted using SAS V.9.4 software (SAS Institute Inc., Kerry, United States).
Results
Patient Characteristics
A total of 1,688 mCRC patients were recruited into the study. More than half (60.2%) were male, with an average age at diagnosis of 57.9 ± 11.57 years. Most patients were married (94.7%), had an education level of <12 years (82.4%), had an annual family income of <100,000 CNY (84.7%), and had medical insurance (98.6%). About half of the patients were treated at an oncology hospital (53.3%). The average HRQOL score prior to the initial treatment was 99.9 ± 20.96. The median duration of mCRC was 10.0 (3.0, 22.0). The liver or lung was the most frequent site of metastases (48.1%) (Table 1).
Utilization of Targeted Therapy
A total of 871 (51.6%) mCRC patients had ever initiated targeted therapy. Distribution of the timing (first-, second-, and third-line) and the kinds of targeted agents first used by mCRC patients are shown in Figure 2A. Of the patients who initiated targeted therapy, 44.5% (388/871), 20.2% (176/871), and 35.2% (307/871) began the medication as a first-, second-, and third-line treatment, respectively. Of those who initiated targeted therapy as the first-line treatment, 65.2% (253/388) and 34.8% (135/388) used bevacizumab and cetuximab, respectively. Of those who first initiated targeted therapy as the second-line treatment, 76.1% (134/176) and 23.9% (42/176) used bevacizumab and cetuximab, respectively. Of those who first initiated targeted therapy as the third-line treatment, 68.1% (209/307), 22.1% (68/307), 6.5% (20/307), and 3.3% (10/307) used bevacizumab, cetuximab, regorafenib, and fruquintinib, respectively. Overall, bevacizumab was the most frequently used targeted agent (68.4%, 596/871), followed by cetuximab (28.1%, 245/871), regorafenib (2.3%, 20/871), and fruquintinib (1.1%, 10/871). These drugs were similarly distributed among patients with an mCRC duration of ≥10 months (Figure 2B).
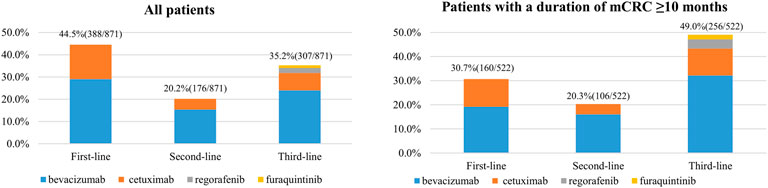
FIGURE 2. Distribution of the timing (first-line, second-line, and third-line) and kinds of targeted agents first used by metastatic CRC (mCRC) patients (China, 2023).
Self-Reported Barriers to the Initiation of Targeted Therapy
Self-reported barriers to the initiation of targeted therapy are shown in Figure 3A. The most common barriers included high medical costs (41.6%, 340/817), lack of belief in the efficacy of targeted therapy (30.8%, 252/817), and a fear of side effects (17.3%, 141/817). The distribution of self-reported barriers was similar among patients with an mCRC duration ≥10 months (Figure 3B).
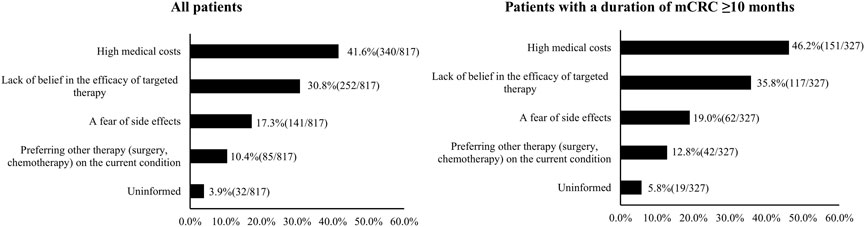
FIGURE 3. Barriers to the initiation of targeted therapy by metastatic CRC (mCRC) patients (China, 2023).
Factors Associated With Initiating Targeted Treatment
The type of hospital, age at mCRC diagnosis, educational level, annual family income, medical insurance, geographic region, HRQOL score prior to first treatment, duration of mCRC, and metastatic site were variables potentially associated with the initiation of targeted therapy and were included in a sequential series of multivariate models (Table 2).
Model 3 was the final multivariate regression model with the lowest AIC. In this final model, patients who were treated in the general hospital (aOR: 0.580, 95% CI: 0.466–0.721), were diagnosed with mCRC at ≥65 years of age (aOR: 0.780, 95% CI: 0.620–0.982), had <7 years of education (aOR: 0.703, 95% CI: 0.494–0.999), had an annual family income <50,000 CNY (aOR: 0.707, 95% CI: 0.501–0.996), had no medical insurance (aOR: 0.203, 95% CI: 0.069–0.593), were located in Southwest China (aOR: 0.684, 95% CI: 0.470–0.994) or Northwest China (aOR: 0.617, 95% CI: 0.237–0.997), had a poor HRQOL score prior to the first mCRC treatment (aOR: 0.729, 95% CI: 0.586–0.907), had an mCRC duration <10 months (aOR: 0.469, 95% CI: 0.382–0.576), or had metastases outside liver/lung or systemic metastasis (aOR: 0.753, 95% CI: 0.606–0.936) were less likely to initiate targeted treatment. Patients who lived in North China (aOR: 1.992, 95% CI: 1.239–3.202), and those with both liver and lung metastases (aOR: 1.614, 95% CI: 1.136–2.295) were more likely to initiate targeted therapy (Table 3).
After limiting the analysis to patients with an mCRC duration
Discussion
This study is the first to provide real-world evidence in support of the use of targeted treatment for mCRC patients in China. The findings illustrated that 51.6% of mCRC patients had ever initiated targeted therapy, one-third of whom first initiated targeted therapy as the third-line treatment. Bevacizumab was the most commonly used medication. High medical cost, lack of belief in the efficacy of targeted therapy and a fear of side effects were the most frequently reported barriers to the initiation of targeted therapy.
While targeted agents have been approved for >10 years, and are recommended by Chinese clinical guidelines, use remains lower than in developed countries. In the United States, for example, >70% of CRC patients with stage IV disease have ever received targeted therapy [29]. The high medical cost was the predominant barrier against initiating targeted agents in this study. In China, the cost for a 1 month’s supply of bevacizumab, cetuximab, regorafenib, fruquintinib is about 9,500 CNY [30], 15,000 CNY [31], 15,000 CNY, and 5,500 CNY [32], respectively. However, more than half of mCRC patients have an annual family income of <50,000 CNY to cover their daily spending needs. While all the targeted agents were listed in the medical insurance directory, and 98.6% of the patients had insurance, the reimbursement rate was limited. In addition, particular limits were added to the cost of treatment during hospitalization. Thus, a cost-reduction strategy may be the first step to facilitate the use of targeted therapy.
Concern about the efficacy and safety of targeted therapy was also a common barrier to treatment initiation. More than one-third of mCRC patients delayed targeted treatment until it became the third-line option, despite studies illustrating that bevacizumab and cetuximab are effective as first- or second-line treatments at improving survival and quality of life [10, 11]. In addition, while regorafenib and fruquintinib have been recommended as the standard third-line mCRC drugs in China since 2017 [19], most patients in the current study still used bevacizumab or cetuximab, suggesting that the misuse of targeted agents is common. Thus, there is a need to improve public awareness of targeted treatments and to strengthen prescriber knowledge of clinical guidelines to promote the timely and correct application of targeted drugs.
Bevacizumab was most frequently used as a first- or second-line drug. In addition to drug cost, the affordability and availability of genetic biomarker testing may also influence the choice of targeted therapy. More than half of advanced CRC patients in the current study had never received biomarker testing due to its high price [33]. Bevacizumab may be an appealing drug choice because it targets VEGF and does not require genetic biomarker testing [21]. However, precision medicine dictates that target drugs are most effective if a predictive biomarker is available and agents that are exempt from biomarker testing should not be selected for use. Biomarkers are also recognized as predictors of targeted drugs and drug reactions and are thus recommended for the selection of personalized therapies by some leading guidelines [6, 34, 35]. For example, cetuximab is associated with a longer OS, a higher objective response rate, a higher complete response, and a greater median depth of response than bevacizumab for the treatment of RAS and BRAF wild-type mCRC [36]. Therefore, policymakers should implement specific strategies, including reducing the cost of testing for key biomarkers and ensuring that they are covered by insurance, to facilitate the use of biomarker tests.
Consistent with patient self-reported barriers to initiating targeted therapy, patients with less education, low annual family income, and no medical insurance were less likely to start targeted treatment. Education level was positively associated with CRC awareness and knowledge [37], thus, less educated patients may not be convinced that targeted therapy is effective. Patients with a low annual family income or no medical insurance may also refuse targeted therapy due to its high expense. Previous studies indicate that elderly patients have limited heath care utilization because they are likely to have less education, lower income, and receive less family support [38, 39]. The current study also found that older patients had a lower probability of using targeted therapy, suggesting that more attention should be paid to this subgroup to ensure equitable cancer care utilization. Slight differences in the use of targeted therapy were also shown by geographic region. Patients who lived in North China had the highest likelihood of initiating targeted therapy, while those living in Southwest or Northwest China had the lowest. North China has a stronger economy, culture, and healthcare system than Southwest and Northwest China, indicating that these factors may be positively associated with the use of targeted therapy. Beijing, which was randomly selected for this survey, is the city with the highest economy, culture, and healthcare services in North China. Thus, this may also have contributed to the high number of patients initiating targeted therapy in this region. These findings highlight that less developed regions should be prioritized for interventions and health education to improve access to targeted treatment.
Patient clinical characteristics were also associated with the likelihood that they would initiate targeted therapy. Those with a shorter duration of mCRC were less likely to initiate targeted therapy. This may be because symptoms were often relatively mild in these patients, suggesting that they may have a higher level of medication hesitation. Thus, more attention and care should be paid to this population. Importantly, patients with more severe disease, including those with low HRQOL scores prior to treatment and those with metastases outside the liver/lung or systemic metastasis, were also less likely to initiate targeted therapy. It is possible that supportive care, including pain management, nutritional support, and psychological intervention may be a better choice for these patients [40, 41]. These findings highlight the need to address targeted treatment hesitation to prevent mCRC from developing to a point at which targeted therapy is no longer an effective option.
Interestingly, patients who were treated in a general hospital were less likely to initiate targeted therapy than those who received treatment in an oncology hospital. This may be because more patients with underlying diseases (such as diabetes, hypertension, and renal insufficiency) or poor organ function that contribute to a poor HRQOL prefer to be treated in a general hospital. Indeed, this study found that patients treated in general hospitals had a lower HRQOL score than those treated in oncology hospitals (94.4 ± 23.12 vs. 104.8 ± 17.42; p < 0.001). Additional data is needed to further assess this hypothesis.
The strengths of this study included its large sample size, multi-center survey nature and hospital-based representative sampling. To our knowledge, this is the first geographically representative study that includes a large number (>1,600) of mCRC patients in China, allowing for a reliable and robust analysis. This study provides real-world evidence for clinicians and policymakers to design interventions that promote the use of targeted therapy and reduce CRC-related mortality.
This study also had several limitations. First, because it is a cross-sectional survey study, causal relationships between patient characteristics and the initiation of targeted treatment cannot be firmly established. Second, the self-reported data may be subject to recall and social desirability biases. Third, since the patients volunteered to enroll, their characteristics may differ from those who did not chose to participate in the study. Finally, only those patients who finished at least the first cycle of chemotherapy were included. The short treatment interval might underestimate the rate of initiating targeted therapy since some patients may delay the use of targeted agents.
Conclusion
This study highlights the limited use of targeted therapy and the frequency of delayed treatment using targeted agents for mCRC patients in China. Bevacizumab was the most frequently used drug. The common barriers to initiating targeted treatment were the high medical cost, the lack of belief in the efficacy of targeted treatment, and a fear of side effects. Reducing the cost of treatment and providing interventional education to improve public awareness are recommended to facilitate the use of targeted therapy.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
This research was approved by the independent review board of Henan Cancer Hospital (2019273). All patients provided written informed consents before participating in this survey.
Author Contributions
YL and XZ contributed to conception and design. J-GZ, S-KZ, and Y-LQ administratively supported this study. H-FX, J-HS, Y-QZ, L-BD, Y-YL, W-JW, H-LC, LM, J-XH, JC, LL, Y-PF, X-FG, C-YF, QZ, X-HW, and J-CD contributed to data curation and data analysis. YL drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Beijing Love Book Cancer Foundation. The authors declare that this study received funding from Merck Serono Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2023.1606091/full#supplementary-material
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi:10.3322/caac.21660
2. Arnold, M, Sierra, MS, Laversanne, M, Soerjomataram, I, Jemal, A, and Bray, F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut (2017) 66(4):683–91. doi:10.1136/gutjnl-2015-310912
3. Yang, Y, Han, Z, Li, X, Huang, A, Shi, J, and Gu, J. Epidemiology and Risk Factors of Colorectal Cancer in China. Chin J Cancer Res (2020) 32(6):729–41. doi:10.21147/j.issn.1000-9604.2020.06.06
4. Zheng, RSZS, Zeng, HM, Wang, SM, Sun, KX, Chen, R, Li, L, et al. Cancer Incidence and Mortality in China, 2016. JNCC (2022) 2(1):2–11. doi:10.1016/j.jncc.2022.02.002
5. Zeng, H, Chen, W, Zheng, R, Zhang, S, Ji, JS, Zou, X, et al. Changing Cancer Survival in China during 2003-15: a Pooled Analysis of 17 Population-Based Cancer Registries. Lancet Glob Health (2018) 6(5):e555–e567. doi:10.1016/S2214-109X(18)30127-X
6. Van Cutsem, E, Nordlinger, B, and Cervantes, A ESMO Guidelines Working Group. Advanced Colorectal Cancer: ESMO Clinical Practice Guidelines for Treatment. Ann Oncol (2010) 21(Suppl. 5):v93–7. doi:10.1093/annonc/mdq222
7. Chibaudel, B, Tournigand, C, Bonnetain, F, Richa, H, Benetkiewicz, M, André, T, et al. Therapeutic Strategy in Unresectable Metastatic Colorectal Cancer: an Updated Review. Ther Adv Med Oncol (2015) 7(3):153–69. doi:10.1177/1758834015572343
8. Geng, F, Wang, Z, Yin, H, Yu, J, and Cao, B. Molecular Targeted Drugs and Treatment of Colorectal Cancer: Recent Progress and Future Perspectives. Cancer Biother Radiopharm (2017) 32(5):149–60. doi:10.1089/cbr.2017.2210
9. Guo, Y, Xiong, BH, Zhang, T, Cheng, Y, and Ma, L. XELOX vs. FOLFOX in Metastatic Colorectal Cancer: An Updated Meta-Analysis. Cancer Invest (2016) 34(2):94–104. doi:10.3109/07357907.2015.1104689
10. Yoshino, T, Arnold, D, Taniguchi, H, Pentheroudakis, G, Yamazaki, K, Xu, RH, et al. Pan-Asian Adapted ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer: a JSMO-ESMO Initiative Endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol (2018) 29(1):44–70. doi:10.1093/annonc/mdx738
11. Loupakis, F, Cremolini, C, Masi, G, Lonardi, S, Zagonel, V, Salvatore, L, et al. Initial Therapy with FOLFOXIRI and Bevacizumab for Metastatic Colorectal Cancer. N Engl J Med (2014) 371(17):1609–18. doi:10.1056/NEJMoa1403108
12. Qin, S, Li, J, Wang, L, Xu, J, Cheng, Y, Bai, Y, et al. Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) versus FOLFOX-4 in Patients with RAS Wild-type Metastatic Colorectal Cancer: The Open-Label, Randomized, Phase III TAILOR Trial. J Clin Oncol (2018) 36(30):3031–9. doi:10.1200/JCO.2018.78.3183
13. Thakur, A, Chorawala, MR, and Patel, RS. A Systemic Review and Meta-Analysis of Aflibercept Plus FOLFIRI Regimen as a Second-Line Treatment for Metastatic Colorectal Cancer: A PRISMA Compliant Pooled Analysis of Randomized Controlled Trials and Single Arm Studies to Assess Efficacy and Safety. Crit Rev Oncol Hematol (2023) 188:104034. doi:10.1016/j.critrevonc.2023.104034
14. Grothey, A, Cutsem, EV, Sobrero, A, Siena, S, Falcone, A, Ychou, M, et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): an International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. The Lancet (2013) 381(9863):303–12. doi:10.1016/S0140-6736(12)61900-X
15. Li, J, Qin, S, Xu, RH, Shen, L, Xu, J, Bai, Y, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients with Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA (2018) 319(24):2486–96. doi:10.1001/jama.2018.7855
16. Chen, J, Wang, J, Lin, H, and Peng, Y. Comparison of Regorafenib, Fruquintinib, and TAS-102 in Previously Treated Patients with Metastatic Colorectal Cancer: A Systematic Review and Network Meta-Analysis of Five Clinical Trials. Med Sci Monit (2019) 25:9179–91. doi:10.12659/MSM.918411
17. Dhillon, S. Regorafenib: A Review in Metastatic Colorectal Cancer. Drugs (2018) 78(11):1133–44. doi:10.1007/s40265-018-0938-y
18.NCCN. Clinical Practice Guidelines in Oncology: colon Cancer V. 4.2008: National Comprehensive Cancer Network, Inc. (2008). Available from: https://www.nccn.org/professionals/physician_gls/PDF/colon.pdf (Accessed January 3, 2023).
19.EgoCccdatn. National Health Commission China Colorectal Cancer Diagnosis and Treatment Norms (2020 Edition). Chin J Gastrointest Surg (2020) 23(6):521–40. doi:10.3760/cma.j.cn115610-20200513-00348
20. Wang, F, Dai, G, Deng, Y, Tang, Y, Wang, W, Niu, Z, et al. Efficacy and Safety of Chemotherapy Combined with Bevacizumab in Chinese Patients with Metastatic Colorectal Cancer: A Prospective, Multicenter, Observational, Non-interventional Phase IV Trial. Chin J Cancer Res (2021) 33(4):490–9. doi:10.21147/j.issn.1000-9604.2021.04.06
21. Rosen, LS, Jacobs, IA, and Burkes, RL. Bevacizumab in Colorectal Cancer: Current Role in Treatment and the Potential of Biosimilars. Target Oncol (2017) 12(5):599–610. doi:10.1007/s11523-017-0518-1
22. Cao, DD, Xu, HL, Xu, XM, and Ge, W. The Impact of Primary Tumor Location on Efficacy of Cetuximab in Metastatic Colorectal Cancer Patients with Different Kras Status: a Systematic Review and Meta-Analysis. Oncotarget (2017) 8(32):53631–41. doi:10.18632/oncotarget.19022
23. Wilhelm, SM, Dumas, J, Adnane, L, Lynch, M, Carter, CA, Schütz, G, et al. Regorafenib (BAY 73-4506): a New Oral Multikinase Inhibitor of Angiogenic, Stromal and Oncogenic Receptor Tyrosine Kinases with Potent Preclinical Antitumor Activity. Int J Cancer (2011) 129(1):245–55. doi:10.1002/ijc.25864
24. Sun, Q, Zhou, J, Zhang, Z, Guo, M, Liang, J, Zhou, F, et al. Discovery of Fruquintinib, a Potent and Highly Selective Small Molecule Inhibitor of VEGFR 1, 2, 3 Tyrosine Kinases for Cancer Therapy. Cancer Biol Ther (2014) 15(12):1635–45. doi:10.4161/15384047.2014.964087
25. Liu, Y, Xu, HF, Zhang, X, Yu, YQ, Zhao, YQ, Zhang, SK, et al. Disease Knowledge, Medical Experience, Health-Related Quality of Life and Health-Care Costs Among Patients with Advanced Colorectal Cancer in China: Protocol for a Nationwide Multicentre Survey. BMJ Open (2022) 12(3):e054403. doi:10.1136/bmjopen-2021-054403
26. Aday, LA, and Andersen, R. A Framework for the Study of Access to Medical Care. Health Serv Res (1974) 9(3):208–20.
27. Andersen, RM. National Health Surveys and the Behavioral Model of Health Services Use. Med Care (2008) 46(7):647–53. doi:10.1097/MLR.0b013e31817a835d
28. Rausa, E, Kelly, ME, Bonavina, L, O'Connell, PR, and Winter, DC. A Systematic Review Examining Quality of Life Following Pelvic Exenteration for Locally Advanced and Recurrent Rectal Cancer. Colorectal Dis (2017) 19(5):430–6. doi:10.1111/codi.13647
29. Neugut, AI, Becker, DJ, Insel, BJ, and Hershman, DL. Uptake of Oxaliplatin and Bevacizumab for Treatment of Node-Positive and Metastatic colon Cancer. J Oncol Pract (2012) 8(3):156–63. doi:10.1200/JOP.2011.000371
30. Zhang, PF, Wen, F, Zhou, J, Huang, JX, Zhou, KX, Wu, QJ, et al. Cost-effectiveness Analysis of Capecitabine Plus Bevacizumab versus Capecitabine Alone in Elderly Patients with Previously Untreated Metastatic Colorectal Cancer from Chinese Societal Perspective. Clin Transl Oncol (2020) 22(1):103–10. doi:10.1007/s12094-019-02114-x
31. Bai, L, Zhang, P, Zhou, K, Liao, W, and Li, Q. Cost-Effectiveness Analysis of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) versus FOLFOX-4 in Patients with RAS Wild-type Metastatic Colorectal Cancer. Cancer Manag Res (2019) 11:10419–26. doi:10.2147/CMAR.S219318
32. Guan, X, Li, H, Xiong, X, Peng, C, Wang, N, Ma, X, et al. Cost-effectiveness Analysis of Fruquintinib versus Regorafenib as the Third-Line Therapy for Metastatic Colorectal Cancer in China. J Med Econ (2021) 24(1):339–44. doi:10.1080/13696998.2021.1888743
33. Zhang, X, Lian, XM, Gu, XF, Liu, Y, Feng, CY, Li, L, et al. Utilization of Genetic Biomarkers Testing and its Associated Factors in Advanced Colorectal Cancer Patients in China: a Nationwide Multicenter Clinical Epidemiological Study. Ann Transl Med (2022) 10(6):324. doi:10.21037/atm-22-988
34. Levine, RA, Chawla, B, Bergeron, S, and Wasvary, H. Multidisciplinary Management of Colorectal Cancer Enhances Access to Multimodal Therapy and Compliance with National Comprehensive Cancer Network (NCCN) Guidelines. Int J Colorectal Dis (2012) 27(11):1531–8. doi:10.1007/s00384-012-1501-z
35. Benson, AB, Venook, AP, Al-Hawary, MM, Arain, MA, Chen, YJ, Ciombor, KK, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2021) 19(3):329–59. doi:10.6004/jnccn.2021.0012
36. Zheng, B, Wang, X, Wei, M, Wang, Q, Li, J, Bi, L, et al. First-line Cetuximab versus Bevacizumab for RAS and BRAF Wild-type Metastatic Colorectal Cancer: a Systematic Review and Meta-Analysis. BMC Cancer (2019) 19(1):280. doi:10.1186/s12885-019-5481-z
37. Xu, HF, Gu, XF, Wang, XH, Wang, WJ, Du, LB, Duan, SX, et al. Knowledge and Awareness of Colorectal Cancer Risk Factors, Screening, and Associated Factors in Advanced Colorectal Cancer Patients: a Multicenter Cross-Sectional Study in China. Ann Transl Med (2022) 10(6):354. doi:10.21037/atm-22-1019
38. Li, C, Tang, C, and Wang, H. Investigating the Association of Health System Characteristics and Health Care Utilization: a Multilevel Model in China's Ageing Population. J Glob Health (2020) 10(2):020802. doi:10.7189/jogh.10.020802
39. Gong, J, Wang, G, Wang, Y, and Zhao, Y. Consumption and Poverty of Older Chinese: 2011-2020. J Econ Ageing (2022) 23:100410. doi:10.1016/j.jeoa.2022.100410
40. Chen, EX, Jonker, DJ, Loree, JM, Kennecke, HF, Berry, SR, Couture, F, et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients with Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol (2020) 6(6):831–8. doi:10.1001/jamaoncol.2020.0910
41. Van Cutsem, E, Peeters, M, Siena, S, Humblet, Y, Hendlisz, A, Neyns, B, et al. Open-label Phase III Trial of Panitumumab Plus Best Supportive Care Compared with Best Supportive Care Alone in Patients with Chemotherapy-Refractory Metastatic Colorectal Cancer. J Clin Oncol (2007) 25(13):1658–64. doi:10.1200/JCO.2006.08.1620
Keywords: associated factors, utilization, barriers, metastatic colorectal cancer, targeted treatment
Citation: Liu Y, Zhang X, Xu H-F, Shi J-H, Zhao Y-Q, Du L-B, Liu Y-Y, Wang W-J, Cao H-L, Ma L, Huang J-X, Cao J, Li L, Fan Y-P, Gu X-F, Feng C-Y, Zhu Q, Wang X-H, Du J-C, Zhang J-G, Zhang S-K and Qiao Y-L (2023) Real-World Utilization, Barriers, and Factors Associated With the Targeted Treatment of Metastatic Colorectal Cancer Patients in China: A Multi-Center, Hospital-Based Survey Study. Int J Public Health 68:1606091. doi: 10.3389/ijph.2023.1606091
Received: 15 April 2023; Accepted: 23 June 2023;
Published: 03 July 2023.
Edited by:
Paolo Chiodini, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Claudia Cardone, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyAnmar Al-Taie, Istinye University, Türkiye
Copyright © 2023 Liu, Zhang, Xu, Shi, Zhao, Du, Liu, Wang, Cao, Ma, Huang, Cao, Li, Fan, Gu, Feng, Zhu, Wang, Du, Zhang, Zhang and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Gong Zhang, emhhbmdqZ0B6enUuZWR1LmNu; Shao-Kai Zhang, c2hhb2thaXpoYW5nQDEyNi5jb20=; You-Lin Qiao, cWlhb3lAY2ljYW1zLmFjLmNu
 Yin Liu
Yin Liu Xi Zhang
Xi Zhang Hui-Fang Xu1
Hui-Fang Xu1