Abstract
Objective: Synthesize longitudinal research evaluating neighborhood environments and cognition to identify methodological approaches, findings, and gaps.
Methods: Included studies evaluated associations between neighborhood and cognition longitudinally among adults >45 years (or mean age of 65 years) living in developed nations. We extracted data on sample characteristics, exposures, outcomes, methods, overall findings, and assessment of disparities.
Results: Forty studies met our inclusion criteria. Most (65%) measured exposure only once and a majority focused on green space and/or blue space (water), neighborhood socioeconomic status, and recreation/physical activity facilities. Similarly, over half studied incident impairment, cognitive function or decline (70%), with one examining MRI (2.5%) or Alzheimer’s disease (7.5%). While most studies used repeated measures analysis to evaluate changes in the brain health outcome (51%), many studies did not account for any type of correlation within neighborhoods (35%). Less than half evaluated effect modification by race/ethnicity, socioeconomic status, and/or sex/gender. Evidence was mixed and dependent on exposure or outcome assessed.
Conclusion: Although longitudinal research evaluating neighborhood and cognitive decline has expanded, gaps remain in types of exposures, outcomes, analytic approaches, and sample diversity.
Introduction
Given the combination of a rapidly aging global population [1] and that Alzheimer’s disease (AD) and related dementias (ADRD) remain incurable conditions [2], research efforts have emphasized identifying risk factors or interventions for healthy aging to prevent cognitive decline and maintain cognitive health. Importantly, racial/ethnic and socioeconomic disparities in AD/ADRD exist and are expected to widen [3]. These factors support the importance of detecting effective interventions for subgroups to achieve health equity.
While research has primarily focused on individual-level interventions for maintaining brain health [4, 5], the neighborhood environment’s role in brain health has become a subject of investigation due to its multiple pathways to health [6]. Neighborhoods may provide important opportunities or obstacles for physical engagement, social interactions, and access to interventions or treatments which could enrich or hinder older adults’ lives and buffer against or accelerate cognitive decline. Two recent systematic reviews provide limited evidence that neighborhood resources are moderately protective of cognitive decline among older adults [7, 8]. Specifically, increased green space/park exposure, community size, and better transportation infrastructure are significantly linked to better cognitive health [8]. Conversely, features of the environment may accelerate progression of AD/ADRD by isolating older adults and limiting access to care, eliciting stress, or containing harmful exposures associated with AD/ADRD, such as air pollution [9]. More critically, perhaps due to declining physical and cognitive function and a diminishing social network, community-dwelling older adults are less likely to leave their immediate environment for work or recreation, making them particularly susceptible to neighborhood effects [10]. Notably, the majority of studies encompassed by previous systematic reviews are cross-sectional in nature, considerably limiting the ability to establish causal relationships between cognitive health and neighborhood factors.
As the area of neighborhood and cognition research has progressed, the importance of longitudinal evidence is increasingly recognized. Longitudinal research offers insight into the causal role of environmental exposures by providing temporality and allowing for the assessment of change in neighborhood conditions [11]. Additionally, it sheds light on AD/ADRD-related progression over time and the mechanisms by which specific features of the environment impact onset of cognitive symptoms. Recent debates regarding modeling of longitudinal research illustrate the complexity of methods necessary to optimize our ability to draw causal inference [12]. Neighborhood data are additionally methodologically unique due to geospatial relationships and opportunities for nesting of observed data [11, 13, 14]. Long-standing methodological obstacles, such as the appropriate size and shape of a neighborhood [15–18], have been investigated in neighborhood cardiovascular research but not for research on cognitive health. Despite these complexities, no existing review has provided an in-depth analysis of longitudinal research or synthesized key methodologies used in this growing field.
Neighborhoods play a role in creating and reinforcing racial/ethnic and socioeconomic disparities in brain health. For example, well-documented geographic patterns of neighborhood resources in the United States show increased access to health-supportive features for predominantly white or wealthy areas [19–21]. Yet the field’s ability to investigate the impact of neighborhoods on cognition for specific subgroups or to understand the interplay between neighborhood disparities and AD/ADRD disparities rests on additional methodologic challenges [22]. Few longitudinal studies have adequate population variability to examine differences by race/ethnicity or socioeconomic status [23]. Even studies with variability may not have adequate sample size to assess effect modification with sufficient precision. This may explain why limited attention to date has focused on the potential modifying effect of factors such as race/ethnicity on the association between neighborhood factors and cognition, despite the evident need for this research [24].
This review aims to systematically assess longitudinal research examining associations between neighborhood characteristics and cognitive outcomes in older adults and to identify the methodological approaches used to account for nested data and estimate change. We extract neighborhood exposures, cognitive outcomes, and the methods employed by this growing field. We also emphasize study population characteristics and potential effect measure modification to detect findings relevant to address racial/ethnic and socioeconomic disparities. Through this systematic literature review and synthesis, we strive to identify existing gaps and guide ongoing and future work.
Methods
Search Strategy
We searched Ovid MEDLINE, PsycInfo, Web of Science, and Embase through 1 July 2022 and supplemented with a reference list from another systematic review [8] (see Supplementary Table S1 for detailed literature search strategy).
Study Selection and Data Extraction
Two reviewers independently reviewed 787 unique citations and 110 full-text articles against a priori inclusion criteria (Figure 1; Supplementary Table S1). Longitudinal analyses of the effect of built or social neighborhood environment on the cognitive function among community-dwelling participants aged (≥45 years or mean age of 65 years) were included. We excluded articles that evaluated environments outside of neighborhoods (e.g., hospitals; assisted living facilities) and those that only evaluated outdoor air/air pollution or COVID-19 pandemic-related changes. To ensure comparability across countries, we excluded studies conducted in countries rated medium or low on the 2020 Human Development Index (HDI). We extracted data on: country, aim of study, population characteristics, length of longitudinal study, neighborhood exposures, spatial unit of analysis, timing of exposure measurement (once, repeated), brain health outcomes, type of longitudinal approach, method to account for correlation, and effect measure modification. Two reviewers independently abstracted data from each included study with any disagreements resolved by consensus or a third reviewer, if necessary. All selection, screening, and extraction was done using Covidence (Melbourne, AUS).
FIGURE 1
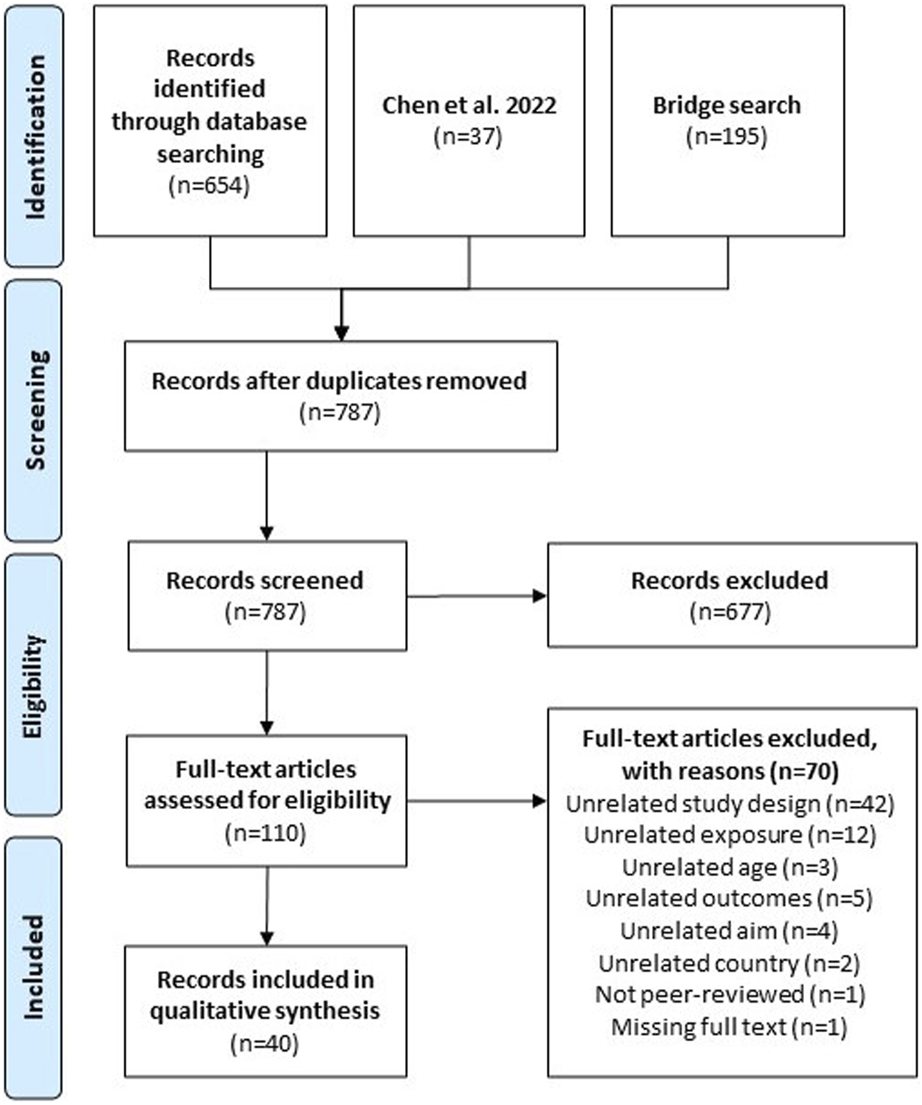
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart of the systematic literature process (performed Philadelphia, United States. 2022).
Article Data Synthesis and Analyses
After extraction of all relevant information, articles were classified by exposure, outcome, and analysis methods. Neighborhood-related exposure measures were categorized into broader topics (Supplementary Table S2) by two neighborhood health experts (JH and YM). Exposures were categorized as single time-point or multiple time-points. Within papers with multiple time-points, type of longitudinal exposure was analyzed (time-varying; cumulative average; change). Cognition-related outcome variables were categorized with input by two neuro-epidemiologists (TH and KH) and one neuropsychologist (Supplementary Table S3). A combination of study design, details, and methods were used to classify papers by analytic strategy. We identified the analytic approach to the longitudinal outcome data (time to event; repeated measure analysis; difference; autoregressive; risk estimation) by reviewing modeling information including statistical model, type of outcome, and whether and how time was used. Additionally, we considered how correlations in exposure or outcomes were accounted for in analyses using nesting or multi-level clusters (none; clustered within geographic area; clustered within individual; both). Papers were considered to evaluate potential effect measure modification of the association between neighborhood and cognition if they included interaction terms and/or reported stratified estimates. We then classified type of effect measure modification based on effect modifier of interest (e.g., race/ethnicity, gender, socioeconomic status). We calculated frequencies of all exposure, outcome, and analysis categories across all papers. The direction of association was also reported and summarized as showing significance, showing no significance, or mixed results.
Results
Included Studies and Populations
The process of study selection is presented as a PRISMA flow diagram in Figure 1. A final count of 40 studies were screened in this literature review. A plurality of the studies were conducted in the United States (43%) [6, 25–40] or China (20%) [41–48] (Supplementary Table S4). Fifty-eight percent of studies included White or European-origin participants [6, 25–35, 37–40, 49–55], 43% included Asian or Pacific Islander participants [25, 26, 33, 36, 41–48, 56–60], and 30% included Black or African American participants [6, 25–32, 34, 39, 40] (Table 1). Half of the studies had only one racial/ethnic background or did not report race [36, 42–50, 52–55, 57–59, 61–63], with only 13% including four or more racial/ethnic groups [25, 26, 31, 34, 39]. Most studies collected environmental exposures through spatial boundaries (47.5%) [6, 25, 28, 29, 34, 35, 38, 46–51, 53, 58–61, 63] or administrative boundaries (37.5%) [26, 27, 30–33, 37, 39, 41, 42, 52, 54, 56, 57, 62], 15% did not specify or used a subjective definition of neighborhood [36, 40, 43–45, 55].
TABLE 1
| Author, year | Country | Total years spanned | Brain health outcomes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical diagnosis | Adjudicated diagnosis | MRI Scans | Verbal learning | Memory | Verbal fluency | Executive function | Global cognitive Score | Attention | Cognitive function/Cognitive decline | Dementia | Alzheimer’s disease | |||
| Astell-Burt, 2020 | Austra-lia | 10 to 14 | X | X | ||||||||||
| Besser, 2021 | United States | 5 to 9 | X | X | X | |||||||||
| Besser, 2022 | United States | 5 to 9 | X | X | X | |||||||||
| Cherrie, 2018 | United Kingdom | 5 to 9 | X | X | ||||||||||
| Cherrie, 2019 | United Kingdom | 5 to 9 | X | X | ||||||||||
| Clarke, 2015 | United States | 15+ | X | X | ||||||||||
| de Keijzer, 2018 | United Kingdom | 10 to 14 | X | X | X | X | X | |||||||
| Fernández-Blázquez, 2021 | Spain | 5 to 9 | X | X | ||||||||||
| Finlay, 2020 | United States | 5 to 9 | X | X | X | X | X | |||||||
| Finlay, 2021 | United States | 10 to 14 | X | X | ||||||||||
| Finlay, 2022 | United States | 10 to 14 | X | X | ||||||||||
| George, 2020 | United States | 15+ | X | X | X | |||||||||
| Ho, 2020 | China | 5 to 9 | X | X | ||||||||||
| Hsu, 2022 | Taiwan | 15+ | X | X | ||||||||||
| Hunt, 2021 | United States | 10 to 14 | X | X | ||||||||||
| Liu, 2020 | Taiwan | <5 | X | X | ||||||||||
| Luo, 2019 | China | <5 | X | X | ||||||||||
| Meyer, 2021 | United States | 5 to 9 | X | X | X | X | ||||||||
| Mobley, 2022 | United States | 15+ | X | X | ||||||||||
| Motohiro, 2021 | Japan | <5 | X | X | ||||||||||
| Ouvrard, 2020 | France | 15+ | X | X | ||||||||||
| Paul, 2020 | Canada | 10 to 14 | X | X | ||||||||||
| Peng, 2022 | China | 5 to 9 | X | X | ||||||||||
| Rodriguez-Loureiro, 2022 | Belgium | 10 to 14 | X | X | ||||||||||
| Sharifian, 2020 | United States | 5 to 9 | X | X | X | |||||||||
| Slawsky, 2022 | United States | 5 to 9 | X | X | ||||||||||
| Tang, 2020 | United States | <5 | X | X | X | X | ||||||||
| Tani, 2019 | Japan | <5 | X | X | ||||||||||
| Vassilaki, 2022 | United States | 10 to 14 | X | X | X | |||||||||
| Wandell, 2020 | Sweden | 10 to 14 | X | X | ||||||||||
| Wang, 2022 | China | 5 to 9 | X | X | X | |||||||||
| Watts, 2015 | United States | <5 | X | X | X | X | X | |||||||
| Weuve, 2021 | United States | 10 to 14 | X | X | X | X | ||||||||
| Worn, 2017 | Netherlands | 5 to 9 | X | X | X | X | ||||||||
| Xiong, 2021 | China | <5 | X | X | ||||||||||
| Yu, 2021 | United States | 5 to 9 | X | X | ||||||||||
| Yuchi, 2020 | Canada | <5 | X | X | ||||||||||
| Zhang, 2022 | China | 5 to 9 | X | X | X | |||||||||
| Zhu, 2019 | China | 10 to 14 | X | X | ||||||||||
| Zhu, 2020 | China | 10 to 14 | X | X | ||||||||||
| Environmental exposures (X = one time point, L = multiple time points) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Environmental hazards | Greenery/Greenspace exposure/Blue exposure | Retail food environment | Neighborhood cohesion | Neighborhood disorder/Aesthetics/Quality | Neighborhood Socioeconomic Status (SES) | Neighborhood Segregation | Social destinations | Public transportation | Walkability | Urbanicity/Rurality Status | Infrastructure | Elevation/Hilliness | Healthcare facilities | Recreation/Physical activity facilities |
| X | ||||||||||||||
| X | ||||||||||||||
| X | ||||||||||||||
| L | ||||||||||||||
| L | ||||||||||||||
| X | X | X | X | |||||||||||
| L | ||||||||||||||
| X | ||||||||||||||
| L | ||||||||||||||
| X | X | |||||||||||||
| X | X | X | X | |||||||||||
| L | ||||||||||||||
| X | X | |||||||||||||
| L | ||||||||||||||
| X | X | X | ||||||||||||
| X | X | X | ||||||||||||
| X | X | X | X | X | X | X | ||||||||
| X | ||||||||||||||
| X | ||||||||||||||
| X | X | X | ||||||||||||
| L | ||||||||||||||
| L | ||||||||||||||
| X | ||||||||||||||
| X | ||||||||||||||
| X | X | |||||||||||||
| X | ||||||||||||||
| L | L | |||||||||||||
| X | ||||||||||||||
| X | ||||||||||||||
| X | ||||||||||||||
| X | ||||||||||||||
| X | X | |||||||||||||
| L | ||||||||||||||
| X | X | |||||||||||||
| X | ||||||||||||||
| X | X | |||||||||||||
| L | L | |||||||||||||
| L | ||||||||||||||
| L | ||||||||||||||
| L | ||||||||||||||
| Analysis type | Effect measure modification (R = race, S=SES, G = gender, N = none) | Multilevel approach | Racial/ethnic makeup | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time to event | Repeated measure | Difference/auto | Risk | None | Individual cluster | Neighborhood cluster | Not specified | White or European | Black or African American | Asian Pacific Islander | Hispanic or latino | Other | |
| X | N | X | X | X | |||||||||
| X | R | X | X | X | X | X | X | ||||||
| X | R, S | X | X | X | X | X | X | ||||||
| X | G, S | X | X | ||||||||||
| X | G | X | X | ||||||||||
| X | N | X | X | X | X | X | |||||||
| X | G, S | X | X | X | |||||||||
| X | S | X | X | ||||||||||
| X | N | X | X | X | X | X | |||||||
| X | N | X | X | X | X | X | |||||||
| X | R, S, G | X | X | X | X | ||||||||
| X | R, S | X | X | X | |||||||||
| X | N | X | X | X | |||||||||
| X | N | X | X | X | X | ||||||||
| X | N | X | X | X | X | X | |||||||
| X | N | X | X | ||||||||||
| X | N | X | X | X | |||||||||
| X | R | X | X | X | X | ||||||||
| X | R | X | X | X | |||||||||
| X | N | X | X | ||||||||||
| X | N | X | X | ||||||||||
| X | S, G | X | X | ||||||||||
| X | S, G | X | X | ||||||||||
| X | S, G | X | X | ||||||||||
| X | N | X | X | X | X | X | |||||||
| X | N | X | X | X | |||||||||
| X | N | X | X | ||||||||||
| X | N | X | X | ||||||||||
| X | G | X | X | X | |||||||||
| X | G | X | X | ||||||||||
| X | S | X | X | ||||||||||
| X | N | X | X | X | |||||||||
| X | R, S | X | X | X | X | X | |||||||
| X | N | X | X | ||||||||||
| X | N | X | X | X | |||||||||
| X | N | X | X | X | X | ||||||||
| X | R, G | X | X | X | |||||||||
| X | N | X | X | X | |||||||||
| X | G, S | X | X | ||||||||||
| X | N | X | X | ||||||||||
Descriptive of included studies.
Neighborhood Exposures and Outcomes
We categorized environmental measures into 15 exposure types (Supplementary Table S2). The most common environmental exposures were green space and/or blue space (water) (30%) [35, 41, 46–48, 51, 53, 57, 60, 61, 63], neighborhood socioeconomic status (SES) (25%) [30, 31, 33, 37, 42, 44, 52, 54, 55, 62] and recreation/physical activity facilities (20%) (Figure 2) [6, 25, 29, 42, 49, 50, 57]. Most studies (65%) only provided environmental exposure measures at one timepoint (Table 1) [6, 25–27, 29, 31–35, 37, 38, 40–45, 53–55, 57–59, 61, 62]. Over half of the papers (70%) examined cognitive function or cognitive decline [6, 25–29, 31, 32, 34, 36–38, 40, 42–51, 53, 55, 56, 58, 60, 62], often through global cognitive score (60%) [6, 25–29, 35, 36, 38–40, 42–51, 55, 56, 58]. Very few papers used magnetic resonance imaging (MRI) data (2.5%) [31] or adjudicated diagnosis of AD/ADRD (10.0%) [30, 33, 37, 52, 54, 57, 59–63]. Symbolizing exposure-outcome pairs (Figure 2) illustrated substantial gaps in evidence. For example, while neighborhood cohesion [34, 36, 38, 40, 43], social destinations [6, 27, 42, 57, 58], and neighborhood disorder [27, 34, 36, 40, 45] were not underrepresented in the overall sample (12.5% of studies had each), none of those papers examined MRI outcomes and only one examined dementia [57].
FIGURE 2
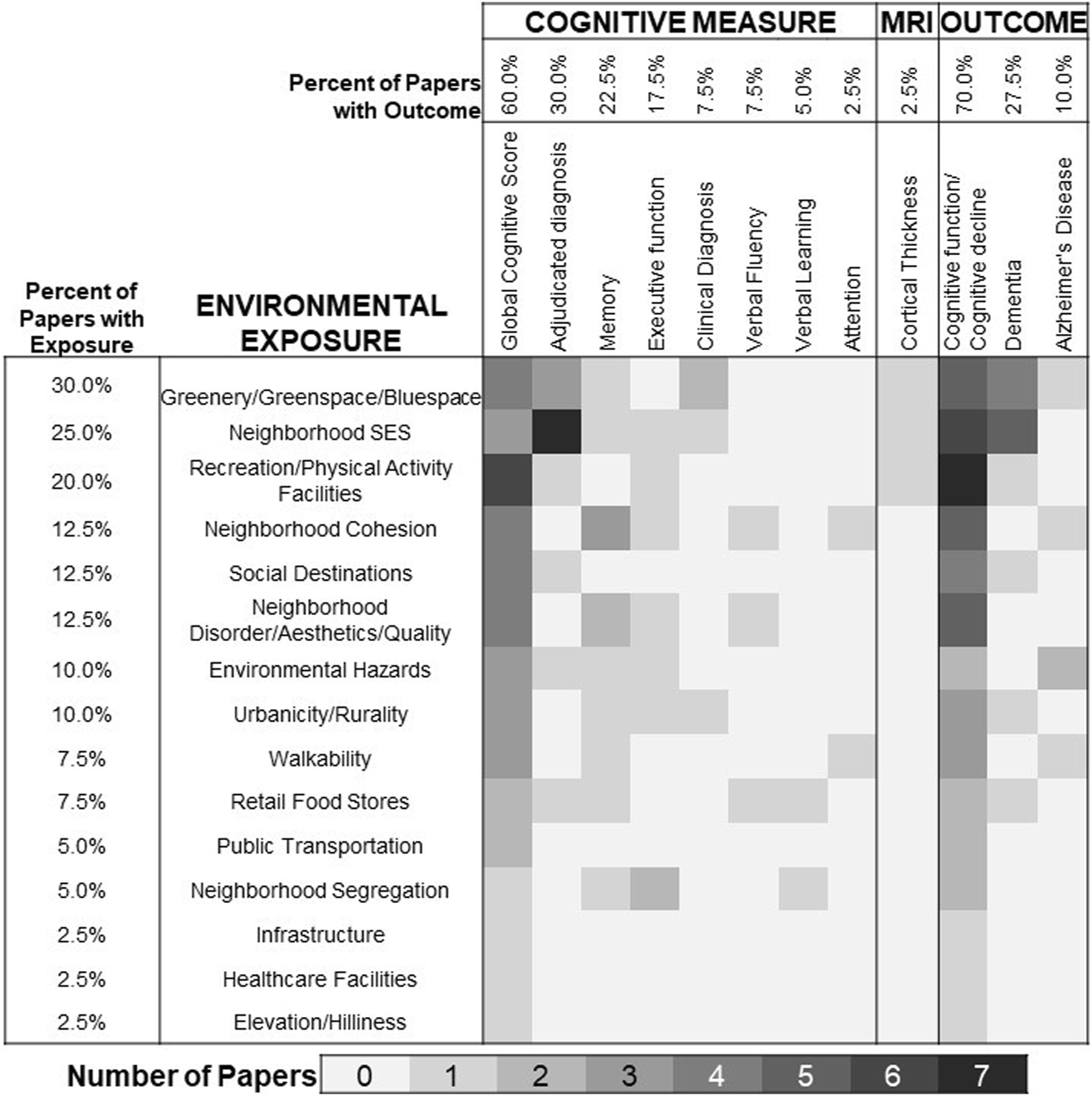
Coverage of environmental exposures and cognitive outcomes in longitudinal research published between 2015 and 2022. Note, cells are not mutually exclusive as many papers examine multiple exposures or multiple outcomes.
Analytic Approach to the Longitudinal Brain Health Data
Over half of the studies used repeated measures analysis to evaluate changes in the brain health outcome (52.5%). A little over a quarter of the studies analyzed the length of time until the occurrence of AD/ADRD (27.5%). A little over ten percent of the studies modeled calculated change or used an auto-regressive model to estimate change in brain health (12.5%). The remaining studies (7.5%) estimated risk or odds of an AD/ADRD event without including time in the calculation.
A third of the studies did not account for any type of correlation in analyses through nesting or multi-level clusters (35%). Another third (30%) accounted for within individual correlation in the outcome (e.g., temporal clustering). The remaining third accounted for clustering of individuals within neighborhood (e.g., spatial clustering) with or without accounting for level-one (individual-level) correlation (35%).
Effect Measure Modification
Less than half (47.5%) of the studies evaluated whether the influence of neighborhood on cognitive outcomes varied by race/ethnicity (20%), SES (30%), and/or gender/sex (n = 27.5%). Of the papers that evaluated race/ethnicity, none identified statistically significant effect measure modification [6, 25, 26, 30, 32, 33, 39, 60]. Regarding effect modification by socioeconomic status, while most studies did not find evidence of significant effect modification [6, 26, 30, 43, 47, 50, 51, 53, 62, 63], two studies suggested the influence of neighborhood characteristics on changes in cognition was stronger among low socioeconomic status participants [39, 44]. One study found the association between park availability and brain health was strongest among women [49]. Another study found that the association between residential surrounding greenness and baseline cognition was stronger for men, but the association between greenness and cognitive decline was stronger for women [51]. None of the other studies evaluating effect measure modification identified a statistically significant effect [6, 37, 43, 47, 50, 53, 54, 60, 63].
Association Between Neighborhood Exposure and Brain Health
The evidence regarding the association between neighborhood exposures and change in brain health is mixed (Table 2). When examining results by type of outcome measure, most analyses that assessed neighborhood factors with MRI scans and verbal fluency displayed an association (100% and 67%, respectively). The exposures most likely to be associated with cognitive change were neighborhood disorder/aesthetics/quality (60%), public transportation (50%), neighborhood cohesion (40%), and social destinations (40%). Among the few studies to examine environmental hazards, walkability, and healthcare facilities, most reported no significant association (50%, 67%, and 100%, respectively). However, the literature investigating neighborhood exposures remains sparse, with few studies evaluating exposures aside from green space/blue space or neighborhood SES.
TABLE 2
| Paper characteristic | Na | Mixed | No association | Association |
|---|---|---|---|---|
| Cognitive Measure | ||||
| Clinical Diagnosis | 3 | 33% (1) | 33% (1) | 33% (1) |
| Adjudicated diagnosis | 11 | 64% (7) | 9% (1) | 27% (3) |
| Verbal Learning | 2 | 50% (1) | 50% (1) | 0% (0) |
| Memory | 9 | 44% (4) | 33% (3) | 22% (2) |
| Verbal Fluency | 3 | 0% (0) | 33% (1) | 67% (2) |
| Executive function | 7 | 43% (3) | 29% (2) | 29% (2) |
| Global Cognitive Score | 24 | 46% (11) | 29% (7) | 25% (6) |
| Attention | 1 | 100% (1) | 0% (0) | 0% (0) |
| MRI Scans | ||||
| Cortical Thickness | 1 | 0% (0) | 0% (0) | 100% (1) |
| Cognitive Outcome | ||||
| Cognitive function/Cognitive decline | 29 | 66% (19) | 7% (2) | 28% (8) |
| Dementia | 11 | 45% (5) | 18% (2) | 36% (4) |
| Alzheimer’s Disease | 3 | 100% (3) | 0% (0) | 0% (0) |
| Neighborhood Exposure | ||||
| Environmental hazards | 4 | 50% (2) | 50% (2) | 0% (0) |
| Greenery/Greenspace exposure/Blue exposure | 10 | 50% (5) | 20% (2) | 30% (3) |
| Retail food environment | 3 | 67% (2) | 33% (1) | 0% (0) |
| Neighborhood cohesion | 5 | 60% (3) | 0% (0) | 40% (2) |
| Neighborhood disorder/Aesthetics/Quality | 5 | 40% (2) | 0% (0) | 60% (3) |
| Neighborhood Socioeconomic Status (SES) | 9 | 33% (3) | 33% (3) | 33% (3) |
| Neighborhood Segregation | 2 | 100% (2) | 0% (0) | 0% (0) |
| Social Destinations | 5 | 20% (1) | 40% (2) | 40% (2) |
| Public Transportation | 2 | 50% (1) | 0% (0) | 50% (1) |
| Walkability | 3 | 0% (0) | 67% (2) | 33% (1) |
| Urbanicity/Rurality Status | 4 | 75% (3) | 0% (0) | 25% (1) |
| Infrastructure | 1 | 100% (1) | 0% (0) | 0% (0) |
| Elevation/Hilliness | 1 | 100% (1) | 0% (0) | 0% (0) |
| Healthcare facilities | 1 | 0% (0) | 100% (1) | 0% (0) |
| Recreation/Physical Activity Facilities | 6 | 33% (2) | 33% (2) | 33% (2) |
| Analysis Type | ||||
| Time to event | 11 | 55% (6) | 18% (2) | 27% (3) |
| Repeated measure | 21 | 71% (15) | 5% (1) | 24% (5) |
| Difference/auto | 5 | 60% (3) | 20% (1) | 20% (1) |
| Risk | 3 | 33% (1) | 0% (0) | 67% (2) |
| Multilevel | ||||
| None | 14 | 57% (8) | 14% (2) | 29% (4) |
| Individual Cluster | 12 | 67% (8) | 0% (0) | 33% (4) |
| Neighborhood Cluster | 3 | 33% (1) | 33% (1) | 33% (1) |
| Both | 11 | 73% (8) | 9% (1) | 18% (2) |
Direction of evidence by exposure, outcome, and analysis type.
Total may not add up to 40 due to some papers examining multiple characteristics.
Discussion
This systematic literature review examined studies on associations between neighborhood characteristics and cognitive outcomes and brain MRI in older adults. While cross-sectional evidence is mounting for these relationships and has been reviewed elsewhere [7, 8] our review focused only on studies that examined longitudinal associations between neighborhood and brain health. Across 15 different neighborhood exposures examined in the research we reviewed, most papers focused on three types of environmental exposures (greenspace, neighborhood SES, and recreation/physical activity facilities) and a majority lacked longitudinal exposure data (i.e., measured environment at only one timepoint and had longitudinal cognitive outcomes). This is consistent with the two previous systematic reviews [7, 8]. Similarly, despite reason to believe that neighborhoods may play a role in creating and reinforcing racial/ethnic and socioeconomic disparities in brain health, we found that the existing longitudinal literature has generally ignored these variations in association or had insufficient diversity of sample to test for effect measure modification. Most research selected appropriate statistical models to analyze longitudinal data but a minority of studies used multi-level modeling to distinguish between neighborhood-level and individual-level influences on changes in brain health.
Our review highlights a need for more longitudinal evidence across a comprehensive set of neighborhood features and outcomes. A large proportion of the papers focused on greenery/greenspace/bluespace (30%), neighborhood SES (25%), and recreation/physical activity facilities (20%). This leaves very limited work on other environmental factors and their pathway to cognition, including neighborhood cohesion, social destinations, neighborhood disorder/aesthetics/quality, environmental hazards, urbanicity/rurality, walkability, retail food stores, public transportation, neighborhood segregation, infrastructure, healthcare facilities, or elevation/hilliness. Greenspace and recreation/physical activity facilities are merely two features of the built environment, while theoretical models propose the existence of potentially synergistic effects stemming from additional features such as third places/destinations, density/land use mix, and connectivity/mobility (e.g., public transportation) [6, 64]. These same models emphasize the importance of neighborhood social environments including safety/crime, disorder, and social connections/cohesion, all of which are currently underrepresented in the longitudinal literature. Our finding of the under-representation of social environments as a risk factor for cognitive aging and dementia is consistent with findings from a recent scoping review which called for the inclusion of time-varying social environmental factors and multiple social ecological levels in future research [65]. Interestingly, these social environment measures may also be more consistently associated with cognitive outcomes; our review showed that neighborhood disorder/aesthetics/quality, neighborhood cohesion, and social destinations were three of the top six exposures significantly associated with change in brain health. Additionally, while it is encouraging to see papers examining underlying fundamental causes of neighborhood features, such as neighborhood SES, more work should be done on additional factors that result in unequal resource distribution such as residential segregation by age or race/ethnicity. Since our review, a few studies evaluating segregation have been published which suggest future research is necessary [26, 66]. Similar gaps and opportunities exist for future work to examine additional measures of brain health. Within our review, 72.5% of papers assessed cognitive function or cognitive decline and 60% focused on Global Cognitive Scores. Very few papers examined indicators such as MRI (2.5%), or clinical outcomes such as all-cause dementia (27.5%) and Alzheimer’s disease (7.5%). MRI scans may be less biased by racial/ethnic, language, and socioeconomic aspects in cognitive testing [67, 68]. This observation becomes particularly relevant as the field strives to understand disparities in brain health and the differential impact of neighborhood factors among different subgroups.
This review identified a growing number of longitudinal studies. Longitudinal studies are critical not just for providing temporality, but also for understanding changes in neighborhood conditions and risk for AD/ADRD. However, a minority of these studies employed multilevel modeling to account for the nesting of individuals within neighborhoods. By using multilevel modeling, researchers can estimate the extent to which group-level factors (e.g., neighborhood characteristics) influence cognition or cognitive events, while controlling for individual-level factors [69]. This approach allows for the investigation of both between-group and within-group variability, as well as the examination of how group-level and individual-level variables are related to individual-level outcomes. Multilevel modeling requires an adequate sample size to detect group-level effects; failure to account for the nested data may reflect insufficient sample to appropriately model the neighborhood influences of interest. Researchers need to conduct sample size and power calculations to ensure that there are enough participants and groups to detect the effects of interest [69], and greater resources and funding should target health-related research at the neighborhood level that use spatial sampling frames to ensure adequate sample size. None of the studies specifically referenced sample size considerations related to their modeling approach.
Most commonly the research we reviewed selected appropriate statistical models, such as mixed models, to analyze longitudinal data with continuous outcomes. These models account for the correlation between repeated assessment of the outcome (e.g., cognitive function) within individuals and the nested structure of the data. However, few studies addressed any baseline imbalance in neighborhood-level factors by using appropriate statistical methods, such as analysis of covariance (ANCOVA) or change models [12]. These methods can help control for confounding and improve the accuracy of estimates. However, studies must clearly define the causal estimates of interest, such as etiological research focused on changes in cognitive function or new events, rather than, for example, health services research focused on burden of disease associated with neighborhood characteristics, to guide the choice of appropriate analysis methods [12]. Further, we did not identify any studies that used alternative analysis methods, such as gain scores or graphical models or marginal structural models controlling for time varying confounder, to assess trajectories of change in cognitive function or cognitive events [70–72]. These methods may provide additional insights into the relationships between neighborhood factors and cognitive outcomes.
In our review, more than half of the studies did not evaluate differences in the association between neighborhood and cognition by key social determinants of health, including race/ethnicity, SES, or gender. Similarly, half of the studies used a single-race or ethnic background sample. Research identifying neighborhood features associated with disparities in cognition are important to ensure that future policy and design-based interventions are carefully planned to reduce inequalities rather than exacerbate them [73]. Risk for AD/ADRD is unequally distributed across gender, SES, and race/ethnicity. African American, Hispanic, low-SES individuals face the highest and most disproportionate risk for AD/ADRD [74–80]. These variations in brain health describe high risk populations but fail to identify specific pathways through which to intervene to reduce disparities. Without diverse cohorts and comprehensive evaluation of the impact of systemic and interpersonal racism, we may be missing key contributors of disparities in brain health. The distribution of neighborhood features and resources varies by neighborhood-level SES and racial composition [21, 81–88]. Relative differences in these neighborhood features may be the clue to deciphering racial/ethnic and SES disparities in AD/ADRD. Consistent with our findings, a recent scoping review of neighborhood influences on racial disparities in cognitive health found that even among studies with diverse samples of racial and ethnic groups, few studies evaluated disparities and those that did used inconsistent approaches to evaluating effect modification by race/ethnicity. Future research, including adequate diversity and attention to outcome measures that are resistant to the biases of traditional cognitive testing, is needed to inform both policy-level, population health interventions and clinician-based interventions tailored to individual patients’ circumstances.
This review is not without its limitations. First, gray literature was not included, suggesting that some articles may have been missed in such a rapidly growing field. Similarly, exclusion of non-English studies restricts the overall generalizability and completeness of this review. Additionally, this review only captured studies conducted in high or very high HDI countries. While the purpose of this exclusion was to make findings more comparable across these countries, results may not be generalizable to less developed settings or countries. Finally, many papers examined multiple exposures (or operationalizations of the same exposure) or outcomes. While we have summarized papers overall and summarized evidence by analyses, this complexity hinders our ability to give a concise or conclusive account of the state of the literature. This lack of consistency across both exposures and outcomes also restricted our ability to perform a quantitative synthesis or estimate statistical combinations of results across studies.
Despite these limitations, this review advances the field’s understanding of the measures, methods, and populations currently represented in the longitudinal literature on neighborhood environments and changes in cognitive status. Specifically, we identified exposure-outcome pairs that warrant more examination, catalogued existing analytic methods including use of multi-level modeling or clustering, explored the inclusion of effect measure modification to understand disparities, and provided an initial summary of the strength of the evidence to guide prioritization of exposures or outcomes. Future work should continue to study both individual neighborhood environment measures and holistic measures of the composite impact of interrelated neighborhood features across the lifespan (e.g., exposome). Analyses should leverage longitudinal data through more complex, advanced methods and modeling techniques. To understand, estimate, and address disparities in cognitive decline, new studies should push for more diverse population samples, robust outcome measures, and analysis that explicitly estimate effects across race/ethnicity and socioeconomic status.
Statements
Author contributions
YM and JH conceived of the project, obtained funding, guided the systematic review, conducted analyses, wrote manuscript drafts, and revised the manuscript. AS, CB, UE, and JL conducted the paper search, reviewed papers for inclusion, performed data extraction, and contributed to the manuscript. TH and KH gave conceptual categories for brain health, reviewed analyses for accuracy, and provided substantive edits to the manuscript. BS gave conceptual categories and statistical guidance on methodology of papers, reviewed analyses for accuracy, and provided substantive edits to the manuscript. LB gave conceptual categories for exposures and outcomes, reviewed analyses for accuracy, and provided substantive edits to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that members of the research team are supported by funding from the National Institute on Aging (R01AG072634) and the Urban Health Collaborative at Drexel University. LB is supported by NIH/NIA K01AG063895, NIH/NIA R21AG075291, and the Alzheimer’s Association (AARG-21-850963). TH and KH are supported by NIH/NIA R01AG058969.
Acknowledgments
We would like to thank Kathleen Turner, Drexel’s Public Health Librarian who helped design the literature review search. We would also like to thank Bonnie Sachs, Neuropsychologist at Wake Forest University for their help in categorizing cognition-related outcome variables.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/phrs.2024.1606677/full#supplementary-material
References
1.
Camici GG Liberale L . Aging: The Next Cardiovascular Disease?Eur Heart J (2017) 38:1621–3. 10.1093/eurheartj/ehx239
2.
World Health Organization. Dementia (2022). Available from: https://www.who.int/news-room/fact-sheets/detail/dementia (Accessed February 29, 2024).
3.
Aranda MP Kremer IN Hinton L Zissimopoulos J Whitmer RA Hummel CH et al Impact of Dementia: Health Disparities, Population Trends, Care Interventions, and Economic Costs. J Am Geriatr Soc (2021) 69:1774–83. 10.1111/jgs.17345
4.
Gavelin HM Dong C Minkov R Bahar-Fuchs A Ellis KA Lautenschlager NT et al Combined Physical and Cognitive Training for Older Adults With and Without Cognitive Impairment: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Ageing Res Rev (2021) 66:101232. 10.1016/j.arr.2020.101232
5.
van Os Y de Vugt ME van Boxtel M . Cognitive Interventions in Older Persons: Do They Change the Functioning of the Brain?Biomed Res Int (2015) 2015:438908–14. 10.1155/2015/438908
6.
Finlay J Esposito M Langa KM Judd S Clarke P . Cognability: An Ecological Theory of Neighborhoods and Cognitive Aging. Soc Sci Med (2022) 309:115220. 10.1016/j.socscimed.2022.115220
7.
Besser LM McDonald NC Song Y Kukull WA Rodriguez DA . Neighborhood Environment and Cognition in Older Adults: A Systematic Review. Am J Prev Med (2017) 53:241–51. 10.1016/j.amepre.2017.02.013
8.
Chen X Lee C Huang H . Neighborhood Built Environment Associated With Cognition and Dementia Risk Among Older Adults: A Systematic Literature Review. Soc Sci Med (2022) 292:114560. 10.1016/j.socscimed.2021.114560
9.
Yen IH Michael YL Perdue L . Neighborhood Environment in Studies of Health of Older Adults. A Systematic Review. Am J Prev Med (2009) 37:455–63. 10.1016/j.amepre.2009.06.022
10.
National Academies of Sciences, Engineering, and Medicine. Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System. Washington, D.C.: National Academies Press (2020).
11.
Diez Roux AV . Estimating Neighborhood Health Effects: The Challenges of Causal Inference in a Complex World. Soc Sci Med (2004) 58:1953–60. 10.1016/S0277-9536(03)00414-3
12.
Glymour MM . Commentary: Modelling Change in a Causal Framework. Int J Epidemiol (2022) 51:1615–21. 10.1093/ije/dyac151
13.
Roux AVD . Investigating Neighborhood and Area Effects on Health. Am J Public Health (2001) 91:1783–9. 10.2105/ajph.91.11.1783
14.
Diez-Roux AV . The Study of Group-Level Factors in Epidemiology: Rethinking Variables, Study Designs, and Analytical Approaches. Epidemiol Rev (2004) 26:104–11. 10.1093/epirev/mxh006
15.
James P Berrigan D Hart JE Aaron Hipp J Hoehner CM Kerr J et al Effects of Buffer Size and Shape on Associations Between the Built Environment and Energy Balance. Health Place (2014) 27:162–70. 10.1016/j.healthplace.2014.02.003
16.
Li J Auchincloss AH Hirsch JA Melly SJ Moore KA Peterson A et al Exploring the Spatial Scale Effects of Built Environments on Transport Walking: Multi-Ethnic Study of Atherosclerosis. Health Place (2022) 73:102722. 10.1016/j.healthplace.2021.102722
17.
Li J Peterson A Auchincloss AH Hirsch JA Rodriguez DA Melly SJ et al Comparing Effects of Euclidean Buffers and Network Buffers on Associations Between Built Environment and Transport Walking: The Multi-Ethnic Study of Atherosclerosis. Int J Health Geogr (2022) 21:12. 10.1186/s12942-022-00310-7
18.
Lovasi GS Grady S Rundle A . Steps Forward: Review and Recommendations for Research on Walkability, Physical Activity and Cardiovascular Health. Public Health Rev (2012) 33:484–506. 10.1007/BF03391647
19.
Richardson LD Norris M . Access to Health and Health Care: How Race and Ethnicity Matter. Mount Sinai J Med (2010) 77:166–77. 10.1002/msj.20174
20.
Gayman MD Wilkin HA Stover S Vidmar CM Edwards T Gallashaw C . Perceived Built Environment and Physical Limitations: Race Contrasts in Historically Lower-Income African American Neighborhoods. Fam Community Health (2021) 44:21–31. 10.1097/FCH.0000000000000282
21.
Bruton CM Floyd MF . Disparities in Built and Natural Features of Urban Parks: Comparisons by Neighborhood Level Race/Ethnicity and Income. J Urban Health (2014) 91:894–907. 10.1007/s11524-014-9893-4
22.
Besser LM Jimenez MP Reimer CJ Meyer OL Mitsova D George KM et al Diversity of Studies on Neighborhood Greenspace and Brain Health by Racialized/Ethnic Group and Geographic Region: A Rapid Review. Int J Environ Res Public Health (2023) 20:5666. 10.3390/ijerph20095666
23.
Mehta KM Yeo GW . Systematic Review of Dementia Prevalence and Incidence in United States Race/ethnic Populations. Alzheimer’s Demen (2017) 13:72–83. 10.1016/j.jalz.2016.06.2360
24.
Beydoun MA Beydoun HA Banerjee S Weiss J Evans MK Zonderman AB . Pathways Explaining Racial/Ethnic and Socio-Economic Disparities in Incident All-Cause Dementia Among Older US Adults Across Income Groups. Transl Psychiatry (2022) 12:478. 10.1038/s41398-022-02243-y
25.
Besser LM Chang LC Evenson KR Hirsch JA Michael YL Galvin JE et al Associations Between Neighborhood Park Access and Longitudinal Change in Cognition in Older Adults: The Multi-Ethnic Study of Atherosclerosis. J Alzheimer’s Dis (2021) 82:221–33. 10.3233/JAD-210370
26.
Besser LM Meyer OL Jones MR Tran D Booker M Mitsova D et al Neighborhood Segregation and Cognitive Change: Multi-Ethnic Study of Atherosclerosis. Alzheimer’s Demen (2022) 19(4):1143–51. 10.1002/alz.12705
27.
Clarke PJ Weuve J Barnes L Evans DA Mendes de Leon CF . Cognitive Decline and the Neighborhood Environment. Ann Epidemiol (2015) 25:849–54. 10.1016/j.annepidem.2015.07.001
28.
Finlay J Esposito M Tang S Gomez-Lopez I Sylvers D Judd S et al Fast-Food for Thought: Retail Food Environments as Resources for Cognitive Health and Wellbeing Among Aging Americans? Health Place (2020) 64:102379. 10.1016/j.healthplace.2020.102379
29.
Finlay J Esposito M Li M Colabianchi N Zhou H Judd S et al Neighborhood Active Aging Infrastructure and Cognitive Function: A Mixed-Methods Study of Older Americans. Prev Med (Baltim) (2021) 150. 10.1016/j.ypmed.2021.106669
30.
George KM Lutsey PL Kucharska-Newton A Palta P Heiss G Osypuk T et al Life-Course Individual and Neighborhood Socioeconomic Status and Risk of Dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. Am J Epidemiol (2020) 189:1134–42. 10.1093/aje/kwaa072
31.
Hunt JFV Vogt NM Jonaitis EM Buckingham WR Koscik RL Zuelsdorff M et al Association of Neighborhood Context, Cognitive Decline, and Cortical Change in an Unimpaired Cohort. Neurology (2021) 96:e2500–12. 10.1212/WNL.0000000000011918
32.
Meyer OL Besser L Mitsova D Booker M Luu E Tobias M et al Neighborhood Racial/ethnic Segregation and Cognitive Decline in Older Adults. Soc Sci Med (2021) 284. 10.1016/j.socscimed.2021.114226
33.
Mobley TM Shaw C Hayes-Larson E Fong J Gilsanz P Gee GC et al Neighborhood Disadvantage and Dementia Incidence in a Cohort of Asian American and Non-Latino White Older Adults in Northern California. Alzheimer’s Demen (2022) 19(1):296–306. 10.1002/alz.12660
34.
Sharifian N Spivey BN Zaheed AB Zahodne LB . Psychological Distress Links Perceived Neighborhood Characteristics to Longitudinal Trajectories of Cognitive Health in Older Adulthood. Soc Sci Med (2020) 258:113125. 10.1016/j.socscimed.2020.113125
35.
Slawsky ED Hajat A Rhew IC Russette H Semmens EO Kaufman JD et al Neighborhood Greenspace Exposure as a Protective Factor in Dementia Risk Among U.S. Adults 75 Years or Older: A Cohort Study. Environ Health (2022) 21:14. 10.1186/s12940-022-00830-6
36.
Tang F Zhang W Chi I Li M Dong XQ . Importance of Activity Engagement and Neighborhood to Cognitive Function Among Older Chinese Americans. Res Aging (2020) 42:226–35. 10.1177/0164027520917064
37.
Vassilaki M Aakre JA Castillo A Chamberlain AM Wilson PM Kremers WK et al Association of Neighborhood Socioeconomic Disadvantage and Cognitive Impairment. Alzheimer’s Demen (2022) 2022:761–70. 10.1002/alz.12702
38.
Watts A Ferdous F Moore KD Burns JM . Neighborhood Integration and Connectivity Predict Cognitive Performance and Decline. Gerontol Geriatr Med (2015) 2015:2333721415599141. 10.1177/2333721415599141
39.
Weuve J D’Souza J Beck T Evans DA Kaufman JD Rajan KB et al Long-Term Community Noise Exposure in Relation to Dementia, Cognition, and Cognitive Decline in Older Adults. Alzheimer’s Demen (2021) 17:525–33. 10.1002/alz.12191
40.
Yu X Yang J Yin Z Jiang W Zhang D . Loneliness Mediates the Relationships Between Perceived Neighborhood Characteristics and Cognition in Middle-Aged and Older Adults. Int J Geriatr Psychiatry (2021) 36:1858–66. 10.1002/gps.5595
41.
Ho HC Fong KNK Chan TC Shi Y . The Associations Between Social, Built and Geophysical Environment and Age-Specific Dementia Mortality Among Older Adults in a High-Density Asian City. Int J Health Geogr (2020) 19:53. 10.1186/s12942-020-00252-y
42.
Luo Y Zhang L Pan X . Neighborhood Environments and Cognitive Decline Among Middle-Aged and Older People in China. Journals Gerontol - Ser B Psychol Sci Soc Sci (2019) 74:e60–71. 10.1093/geronb/gbz016
43.
Peng C Han SH Burr JA . Perceptions of Childhood Neighborhood Social Cohesion and Cognitive Function in Middle and Late Adulthood. Gerontologist (2022) 62:1266–77. 10.1093/geront/gnac022
44.
Wang Y Jiang Y Wu W Xu K Zhao Q Tan Z et al Education, Neighborhood Environment, and Cognitive Decline: Findings From Two Prospective Cohort Studies of Older Adults in China. Alzheimer’s Demen (2022) 19:560–8. 10.1002/alz.12679
45.
Xiong P Liang X Chen H Chen L Zuo L Jing C et al Association Between Childhood Neighborhood Quality and the Risk of Cognitive Dysfunction in Chinese Middle-Aged and Elderly Population: The Moderation Effect of Body Mass Index. Front Aging Neurosci (2021) 13:645189. 10.3389/fnagi.2021.645189
46.
Zhang L Luo Y Zhang Y Pan X Zhao D Wang Q . Green Space, Air Pollution, Weather, and Cognitive Function in Middle and Old Age in China. Front Public Health (2022) 10:871104. 10.3389/fpubh.2022.871104
47.
Zhu A Wu C Yan LL Wu C-D Bai C Shi X et al Association Between Residential Greenness and Cognitive Function: Analysis of the Chinese Longitudinal Healthy Longevity Survey. BMJ Nutr Prev Health (2019) 2:72–9. 10.1136/bmjnph-2019-000030
48.
Zhu A Yan L Shu C Zeng Y Ji JS . APOE ε4 Modifies Effect of Residential Greenness on Cognitive Function Among Older Adults: A Longitudinal Analysis in China. Sci Rep (2020) 10:82. 10.1038/s41598-019-57082-7
49.
Cherrie MPC Shortt NK Mitchell RJ Taylor AM Redmond P Thompson CW et al Green Space and Cognitive Ageing: A Retrospective Life Course Analysis in the Lothian Birth Cohort 1936. Soc Sci Med (2018) 196:56–65. 10.1016/j.socscimed.2017.10.038
50.
Cherrie MPC Shortt NK Thompson CW Deary IJ Pearce JR . Association Between the Activity Space Exposure to Parks in Childhood and Adolescence and Cognitive Aging in Later Life. Int J Environ Res Public Health (2019) 16:632. 10.3390/ijerph16040632
51.
de Keijzer C Tonne C Basagaña X Valentín A Singh-Manoux A Alonso J et al Residential Surrounding Greenness and Cognitive Decline: A 10-Year Follow-Up of the Whitehall II Cohort. Environ Health Perspect (2018) 126:077003. 10.1289/EHP2875
52.
Ouvrard C Meillon C Dartigues JF Ávila-Funes JA Amieva H . Do Individual and Geographical Deprivation Have the Same Impact on the Risk of Dementia? A 25-Year Follow-Up Study. Journals Gerontol (2020) 75:218–27. 10.1093/geronb/gbx130
53.
Rodriguez-Loureiro L Gadeyne S Bauwelinck M Lefebvre W Vanpoucke C Casas L . Long-Term Exposure to Residential Greenness and Neurodegenerative Disease Mortality Among Older Adults: A 13-Year Follow-Up Cohort Study. Environ Health (2022) 21:49. 10.1186/s12940-022-00863-x
54.
Wändell P Carlsson AC Li X Gasevic D Sundquist J Sundquist K . The Association Between Sociodemographic Characteristics and Dementia in Patients With Atrial Fibrillation. Aging Clin Exp Res (2020) 32:2319–27. 10.1007/s40520-019-01449-3
55.
Wörn J Ellwardt L Aartsen M Huisman M . Cognitive Functioning Among Dutch Older Adults: Do Neighborhood Socioeconomic Status and Urbanity Matter?Soc Sci Med (2017) 187:29–38. 10.1016/j.socscimed.2017.05.052
56.
Hsu HC Bai CH . Individual and Environmental Factors Associated With Cognitive Function in Older People: A Longitudinal Multilevel Analysis. BMC Geriatr (2022) 22:243. 10.1186/s12877-022-02940-9
57.
Liu CC Sun Y Kung SF Kuo HW Huang NC Li CY et al Effects of Physical and Social Environments on the Risk of Dementia Among Taiwanese Older Adults: A Population-Based Case-Control Study. BMC Geriatr (2020) 20:226. 10.1186/s12877-020-01624-6
58.
Motohiro A Abe T Okuyama K Onoda K Ito T Isomura M et al Environmental Factors Affecting Cognitive Function Among Community-Dwelling Older Adults: A Longitudinal Study. Int J Environ Res Public Health (2021) 18:8528. 10.3390/ijerph18168528
59.
Tani Y Suzuki N Fujiwara T Hanazato M Kondo K . Neighborhood Food Environment and Dementia Incidence: The Japan Gerontological Evaluation Study Cohort Survey. Am J Prev Med (2019) 56:383–92. 10.1016/j.amepre.2018.10.028
60.
Yuchi W Sbihi H Davies H Tamburic L Brauer M . Road Proximity, Air Pollution, Noise, Green Space and Neurologic Disease Incidence: A Population-Based Cohort Study. Environ Health (2020) 19:8. 10.1186/s12940-020-0565-4
61.
Astell-Burt T Navakatikyan MA Feng X . Urban Green Space, Tree Canopy and 11-Year Risk of Dementia in a Cohort of 109,688 Australians. Environ Int (2020) 145:106102. 10.1016/j.envint.2020.106102
62.
Fernández-Blázquez MA Noriega-Ruiz B Ávila-Villanueva M Valentí-Soler M Frades-Payo B Del Ser T et al Impact of Individual and Neighborhood Dimensions of Socioeconomic Status on the Prevalence of Mild Cognitive Impairment Over Seven-Year Follow-Up. Aging Ment Health (2021) 25:814–23. 10.1080/13607863.2020.1725803
63.
Paul LA Hystad P Burnett RT Kwong JC Crouse DL van Donkelaar A et al Urban Green Space and the Risks of Dementia and Stroke. Environ Res (2020) 186:109520. 10.1016/j.envres.2020.109520
64.
Schulz A Northridge ME . Social Determinants of Health: Implications for Environmental Health Promotion. Health Edu Behav (2004) 31:455–71. 10.1177/1090198104265598
65.
Peterson RL George KM Tran D Malladi P Gilsanz P Kind AJH et al Operationalizing Social Environments in Cognitive Aging and Dementia Research: A Scoping Review. Int J Environ Res Public Health (2021) 18:7166. 10.3390/ijerph18137166
66.
Meyer OL Besser L Tobias M George KM Gavett B Farias ST et al Neighborhood Socioeconomic Status and Segregation Linked to Cognitive Decline. Alzheimer’s Demen Diagn Assess Dis Monit (2023) 15:e12401. 10.1002/dad2.12401
67.
Fyffe DC Mukherjee S Barnes LL Manly JJ Bennett DA Crane PK . Explaining Differences in Episodic Memory Performance Among Older African Americans and Whites: The Roles of Factors Related to Cognitive Reserve and Test Bias. J Int Neuropsychological Soc (2011) 17:625–38. 10.1017/S1355617711000476
68.
Aiken Morgan AT Marsiske M Dzierzewski JM Jones RN Whitfield KE Johnson KE et al Race-Related Cognitive Test Bias in the Active Study: A Mimic Model Approach. Exp Aging Res (2010) 36:426–52. 10.1080/0361073X.2010.507427
69.
Diez Roux AV . The Study of Group-Level Factors in Epidemiology: Rethinking Variables, Study Designs, and Analytical Approaches. Epidemiol Rev (2004) 104–11. 10.1093/epirev/mxh006
70.
Wright DB . Gain Scores, ANCOVA, and Propensity Matching Procedures for Evaluating Treatments in Education. Open Edu Stud (2020) 2:45–65. 10.1515/edu-2020-0107
71.
Bhushan N Mohnert F Sloot D Jans L Albers C Steg L . Using a Gaussian Graphical Model to Explore Relationships Between Items and Variables in Environmental Psychology Research. Front Psychol (2019) 10:1050. 10.3389/fpsyg.2019.01050
72.
Caunca MR Odden MC Glymour MM Elfassy T Kershaw KN Sidney S et al Association of Racial Residential Segregation Throughout Young Adulthood and Cognitive Performance in Middle-Aged Participants in the CARDIA Study. JAMA Neurol (2020) 77:1000–7. 10.1001/jamaneurol.2020.0860
73.
Zhang CQ Chung PK Zhang R Schüz B . Socioeconomic Inequalities in Older Adults’ Health: The Roles of Neighborhood and Individual-Level Psychosocial and Behavioral Resources. Front Public Health (2019) 7:318. 10.3389/fpubh.2019.00318
74.
Matthews KA Xu W Gaglioti AH Holt JB Croft JB Mack D et al Racial and Ethnic Estimates of Alzheimer’s Disease and Related Dementias in the United States (2015–2060) in Adults Aged ≥65 Years. Alzheimer’s Demen (2019) 15:17–24. 10.1016/j.jalz.2018.06.3063
75.
Mayeda ER Glymour MM Quesenberry CP Whitmer RA . Inequalities in Dementia Incidence Between Six Racial and Ethnic Groups Over 14 Years. Alzheimer’s Demen (2016) 12:216–24. 10.1016/j.jalz.2015.12.007
76.
Sattler C Toro P Schönknecht P Schröder J . Cognitive Activity, Education and Socioeconomic Status as Preventive Factors for Mild Cognitive Impairment and Alzheimer’s Disease. Psychiatry Res (2012) 196:90–5. 10.1016/j.psychres.2011.11.012
77.
Mortimer JA Graves AB . Education and Other Socioeconomic Determinants of Dementia and Alzheimer’s Disease. Neurology-Minneapolis (1993) 43:39–39.
78.
Maresova P Mohelska H Dolejs J Kuca K . Socio-Economic Aspects of Alzheimer’s Disease. Curr Alzheimer Res (2015) 12:903–11. 10.2174/156720501209151019111448
79.
Mayeda ER . Invited Commentary: Examining Sex/Gender Differences in Risk of Alzheimer Disease and Related Dementias—Challenges and Future Directions. Am J Epidemiol (2019) 188:1224–7. 10.1093/aje/kwz047
80.
Adkins-Jackson PB George KM Besser LM Hyun J Lamar M Hill‐Jarrett TG et al The Structural and Social Determinants of Alzheimer’s Disease Related Dementias. Alzheimer’s Demen (2023) 19:3171–85. 10.1002/alz.13027
81.
Jacobs J Alston L Needham C Backholer K Strugnell C Allender S et al Variation in the Physical Activity Environment According to Area-Level Socio-Economic Position—A Systematic Review. Obes Rev (2019) 20:686–700. 10.1111/obr.12818
82.
Larson NI Story MT Nelson MC . Neighborhood Environments: Disparities in Access to Healthy Foods in the U.S. Am J Prev Med (2009) 36:74–81. 10.1016/j.amepre.2008.09.025
83.
Moore LV Diez Roux AV . Associations of Neighborhood Characteristics With the Location and Type of Food Stores. Am J Public Health (2006) 96:325–31. 10.2105/AJPH.2004.058040
84.
Hirsch JA Green GF Peterson M Rodriguez DA Gordon-Larsen P . Neighborhood Sociodemographics and Change in Built Infrastructure. J Urban (2017) 10:181–97. 10.1080/17549175.2016.1212914
85.
Hirsch JA Grengs J Schulz A Adar SD Rodriguez DA Brines SJ et al How Much Are Built Environments Changing, and Where? Patterns of Change by Neighborhood Sociodemographic Characteristics Across Seven U.S. Metropolitan Areas. Soc Sci Med (2016) 169:97–105. 10.1016/j.socscimed.2016.09.032
86.
Tsui J Hirsch JA Bayer FJ Quinn JW Cahill J Siscovick D et al Patterns in Geographic Access to Health Care Facilities Across Neighborhoods in the United States Based on Data From the National Establishment Time-Series Between 2000 and 2014. JAMA Netw Open (2020) 3:e205105. 10.1001/jamanetworkopen.2020.5105
87.
Hirsch JA Zhao Y Melly S Moore KA Berger N Quinn J et al National Trends and Disparities in Retail Food Environments in the USA Between 1990 and 2014. Public Health Nutr (2023) 26:1052–62. 10.1017/S1368980023000058
88.
Sullivan A Armendariz M Thierry AD . A Scoping Review of Neighborhoods and Cognitive Health Disparities Among US Midlife and Older Adults. J Aging Health (2023) 36:257–70. 10.1177/08982643231185379
Summary
Keywords
longitudinal study, neighborhoods, cognition, adults, aging, Alzheimer’s disease
Citation
Michael YL, Senerat AM, Buxbaum C, Ezeanyagu U, Hughes TM, Hayden KM, Langmuir J, Besser LM, Sánchez B and Hirsch JA (2024) Systematic Review of Longitudinal Evidence and Methodologies for Research on Neighborhood Characteristics and Brain Health. Public Health Rev 45:1606677. doi: 10.3389/phrs.2024.1606677
Received
27 September 2023
Accepted
20 February 2024
Published
26 March 2024
Volume
45 - 2024
Edited by
Ana Ribeiro, University Porto, Portugal
Reviewed by
Nicole Geovana Dias, Federal University of Uberlândia (UFU), Brazil
Virgínia Conceição, University Porto, Portugal
Renee Zahnow, The University of Queensland, Australia
Updates
Copyright
© 2024 Michael, Senerat, Buxbaum, Ezeanyagu, Hughes, Hayden, Langmuir, Besser, Sánchez and Hirsch.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms. PHR is edited by the Swiss School of Public Health (SSPH+) in a partnership with the Association of Schools of Public Health of the European Region (ASPHER)+
*Correspondence: Yvonne L. Michael, ylm23@drexel.edu
This Review is part of the PHR Special Issue “Neighbourhood Influences on Population Health”
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.