Abstract
Objectives:
Tuberculosis (TB) is a significant global health issue, especially for children living with HIV/AIDS. Hence, the objective of this study was to determine the incidence of TB among children on Anti-retroviral treatment (ART) and its predictors in Northwest Ethiopia.
Methods:
A retrospective follow-up study was conducted among 428 children on ART using simple random sampling from patient registries (2011–2020). STATA statistical software was used for data analysis. The Cox regression model was used to explore predictors of TB infection.
Result:
The study found that the incidence density of TB was 3.37 cases per 100 person-years. The risk factors for TB incidence among children on ART included a history of contact with active TB cases, missed isoniazid preventive therapy, advanced HIV/AIDS stages according to WHO clinical staging, poor drug adherence, and incomplete vaccination status.
Conclusion:
The incidence of TB among children on ART is high, particularly within the first year of enrollment. Children with incomplete vaccination, poor adherence, missed isoniazid prophylaxis, a history of TB contact, and advanced WHO clinical stage are at an increased risk of TB incidence.
Introduction
Tuberculosis (TB) is a bacterial disease caused by the bacterium Mycobacterium tuberculosis. Although it is the most preventable kind of disease in the world, it is nevertheless a significant burden of disease in children [1, 2]. It is the primary cause of death in all HIV-positive individuals, which include children and adolescents [3, 4]. Of the 10 million new cases of tuberculosis, 8.6% involved people living with HIV/AIDS (PLWHA). HIV co-infection and tuberculosis co-infection are terms used to describe the co-infections of the two diseases. A person who has HIV infection and an untreated latent TB infection is much more likely to have TB than a person without HIV infection [5]. Tuberculosis infection can be detected by many techniques, such as culture from biological specimens, acid-fast bacilli microscopy, or bacteriologic confirmation if the TB bacilli are identified by Gene Xpert. Assuming that two of the three following pieces of evidence are satisfied, a structured algorithmic approach can be used to diagnose tuberculosis in young children: The presence of TB-related clinical characteristics, TB contact details, and TB-related alterations on chest radiographs. Furthermore, a child’s radiological picture of a miliary pattern, histopathological results consistent with tuberculosis, or the presence of clinical signs and symptoms could all be used to diagnose TB in young children. Nonetheless, it is still very difficult to diagnose tuberculosis in children living with HIV in underdeveloped countries [1, 6, 7].
Every HIV-infected child should be clinically examined for TB at every healthcare facility visit. The goal of this evaluation should be to identify individuals who are likely to have Tuberculosis and require anti-TB medication, in addition to those who should begin Isoniazid Preventive Therapy (IPT) [8–10].
Healthcare professionals may fail to perform or delay a clinical examination when a patient presents with symptoms suggestive of tuberculosis (TB) [11]. The likelihood of mortality and transmission of infection in the home, community, and medical settings may increase with delayed diagnosis of tuberculosis [12, 13]. Many individuals do not show typical symptoms in the early stages of active TB. Thus, early case detection and screening are advantageous [14–16].
Due to the high prevalence of tuberculosis (TB), a significant amount of work will be required to achieve the Sustainable Development Goals (SDGs) and END-TB plans [17, 18]. Although there has been progress, it is anticipated that the End TB Strategy’s goal of eradicating tuberculosis may not be achieved [19, 20]. Although the goal was to reduce the incidence of tuberculosis by 20% between 2015 and 2020, the global 2020 TB report revealed that tuberculosis decreased by only 9% during this period, or approximately 2% annually. Similarly, only a 14% decrease in mortality was observed between 2015 and 2020, falling short of the 35% target [13, 21, 22].
Childhood TB is often ignored due to its many nonspecific symptoms that overlap with other prevalent pediatric infections. However, young children are more likely to have severe tuberculosis, which has a higher mortality rate [4, 23]. Furthermore, compared to older children and adults, neonates and young children are more prone to experience life-threatening forms of TB disease (disseminated TB, TB meningitis) [18, 24, 25].
To meet the targets of the WHO 2030 “End TB” strategy and the goal of eradicating TB and HIV by 2035, TB programs must collaborate with HIV/AIDS patients to ensure sufficient prophylaxis and ART [22, 26–28].
Only a few previous studies have been conducted, despite the fact that the issue still exists in Ethiopia. The majority of these studies have reported inconsistent median times for developing tuberculosis reports and did not concentrate on survival time to tuberculosis development [29–31]. In particular, no research has been conducted in the current study area. As a result, the purpose of this study was to ascertain the prevalence of tuberculosis among children receiving antiretroviral therapy (ART) and its predictors in Northwest Ethiopia.
Methods
A retrospective follow-up study design was conducted in Bahir Dar city from patient registries from 1 Jan 2011 to 30 Dec 2020. Data were collected from May 01–30/2021. Bahir Dar city is the administrative capital city of Amhara Regional State and is located 552 km northwest of Addis Ababa, Ethiopia. The city has two public referral Hospitals, one public primary hospital and six health centers. Of these, one referral hospital, one primary hospital, and six health centers provide ART services. According to the Amhara Regional Health Bureau report in 2021, there were 913 children on ART in Bahir Dar city.
Source and Study Population
The study included children aged 0–15 living with HIV who initiated ART at selected public health facilities between January 1, 2011, and December 30, 2020, and received treatment for ≥3 months. Exclusions applied to patients lacking baseline data (e.g., socio-demographics, TB infection timing post-ART initiation, or incomplete start/exit dates). Whereas patients’ charts with missed baseline information (at least the child’s socio-demographics, unknown time of TB infection after treatment invitation, start date, and end date of follow-up) were excluded.
Dependent Variable
Time to TB development at any time t (event = 1 and censored = 0).
Independent Variables: Age, sex, maternal serostatus at delivery, place of residence, Caregiver type, parental status, family size, treatment failure, viral load, PMCT, clinical stage, CD4 count, Hgb count/anemic status, opportunistic infection, isoniazid preventive therapy, adherence to ART drugs, cotrimoxazole preventive therapy, date of ART initiation, change in ART regimen related to TB, TB contact history, previous TB treatment history, child vaccination status, body mass index.
Operational Definitions
Active Tuberculosis: Refers to a patient whose diagnosis of tuberculosis has been bacteriologically confirmed or diagnosed by a clinician’s decision.
Incidence of Tuberculosis: Refers to the diagnosis of new cases of tuberculosis in children on ART using bacteriological examination (smear microscopy, TB culture, and Gene Xpert MTB/RIF assay), imaging techniques (Chest radiograph), and histopathology or biochemical analysis of body parts/fluids”.
CD4 count: This is a test that determines the number of CD4 cells in the blood. A CD4 count of less than 350 is considered below the threshold.
Opportunistic infections: Any of the following diseases: Bacterial pneumonia, oral ulcers, Herpes zoster, Pneumocystis carinii pneumonia (PCP), chronic or acute diarrhea, toxoplasmosis of the central nervous system, and Meningitis caused by streptococcal bacteria in HIV-infected children.
Isoniazid preventive therapy: Chemo-prophylaxis used to reduce the risk of developing TB [18].
Cotrimoxazole preventive therapy: Chemo-prophylaxis given to reduce the risk of opportunistic infections [18].
Vaccination status: Children who receive all appropriate vaccines correlated with their age.
TB contact History: Children receiving HIV/AIDS treatment and had a history of contact with active tuberculosis patients before they develop tuberculosis [13].
Events: The outcome of interest, in this case, is the development of tuberculosis in children receiving ART.
Censored: Children who were either lost to follow-up, dropped out and transferred before developing TB, died due to other causes before the end of follow-up or completed the study period before developing tuberculosis were considered censored.
Level of ART Adherence
Good Level of ART adherence: Children with a score of ≥95% or <2 missed doses per month or <3 missed doses per 2 months were considered as having a good level of adherence.
Fair level of ART adherence: children with a score of 85%–94% or 3–5 missed doses per 30 doses or 3–9 missed doses per 60 doses.
Poor level of ART adherence: children with a score of less than 85% or >6 missed doses per 30 doses or >9 missed doses per 60 doses.
Sample Size Determination
The sample size for this study was determined using STATA version 14 with the following assumptions: 95% CI, Power = 85, ratio = 1:1, event among unexposed = 12.87%, AHR = 1.99 (functional status) and a 10% non-response rate. Thus, the final sample size was 428 children.
Sampling Technique and Sampling Procedure
Two public hospitals, namely Felegehiwot Comprehensive Referral Hospital and Adisalem Primary Hospital, and six public health centers, namely Bahir Dar Health Center, Hane Health Center, Abay Health Center, Dagmawi Minilik Health Center, Tis Abay Health Center, and Shimbit were included. The sample size was then allocated proportionally to the patient load of each healthcare facility. As a sampling frame, a list of children on ART was obtained from each health facility using the patients’ computerized medical record numbers. Study participants were recruited from public health facilities: Felegehiwot Comprehensive Specialized Hospital, Adisalem Primary Hospital, Bahir Dar Health Center, Hane Health Center, Abay Health Center, Dagmawi Minilik Health Center, Tis Abay Health Center, and Shimbit.
Data Collection Tools and Procedure
The data were collected using a data extraction checklist. The starting time for each study participant began from the date of ART initiation. The total follow-up for this study was 10 years. The event was the incidence of Tuberculosis following the start of ART, with the condition diagnosed using bacteriologic examination (smear microscopy, TB culture, and Gene Xpert MTB/RIF assay), imaging techniques (Chest radiograph), and histopathological or biochemical analysis of body parts/fluids. Before the actual data collection, four nurse data collectors and one BSc nurse supervisor were trained for half a day on the objectives and data collection procedures of the study.
Data Quality Management
Supervisors and data collectors were trained for half a day. Before data entry, the collected data were reviewed and checked for completeness.
Data Processing and Analysis Procedure
The data were entered using Epi data version 3.1 and then exported to STATA version 14 for analysis. Descriptive analysis was performed to characterize the proportion of socio-demographic, baseline clinical characteristics and treatment-related variables. The results of the study variables were presented using text, tables and figures. The Kaplan-Meier plot was used to estimate the probability of survival time. The life table was used to estimate TB incidence for each subsequent time interval. The proportional hazards assumption was tested graphically, and the global goodness-of-fit test was tested statistically. A bivariable Cox proportional regression model was constructed and those variables having a p-value <0.25 were included in the multivariable Cox proportional hazards regression model. Thus, variables with a P-value <0.05 with 95% CI were considered significant predictors of time to Tuberculosis development. Furthermore, the Cox-Snell residual plot was used to assess the goodness-of-fit of the Cox proportional hazards regression model.
Results
Socio-Demographic Characteristics
Following a review of the records of 428 ART children, 415 were included in the final analysis. Thirteen records with missing baseline information (at least the child’s socio-demographic characteristics, unknown time of TB infection after treatment invitation, start date and end date of follow-up) were excluded. In this study, 53.73% of the subjects were male children and 75.9% of them were urban residents (Table 1).
TABLE 1
| Variable | Category of variables | Outcome | Frequency | Percent (%) | |
|---|---|---|---|---|---|
| Event | Censored | ||||
| Sex of child | Male | 27 | 196 | 223 | 53.73 |
| Female | 33 | 159 | 192 | 46.27 | |
| Age of child in years | <5 | 27 | 146 | 173 | 41.68 |
| 5–10 | 24 | 145 | 169 | 40.72 | |
| 10–15 | 9 | 64 | 73 | 17.60 | |
| Place of residence of the child | Urban | 47 | 268 | 315 | 75.9 |
| Rural | 13 | 87 | 100 | 24.1 | |
| Family size | 2–4 (normal) | 44 | 272 | 316 | 76.14 |
| ≥5 (numerous) | 16 | 83 | 99 | 23.86 | |
| Parental status | Both alive | 18 | 171 | 189 | 45.54 |
| Mother/father dead | 24 | 131 | 155 | 37.35 | |
| Both dead | 18 | 53 | 71 | 17.11 | |
| Caregiver | Parents | 36 | 299 | 335 | 80.72 |
| Sibling | 13 | 28 | 41 | 9.88 | |
| Grandparent | 6 | 20 | 26 | 6.27 | |
| Orphan center | 5 | 8 | 13 | 3.13 | |
Baseline socio-demographic characteristics of children on Anti-retroviral treatment in Bahir Dar City, Northwest Ethiopia, 2021 (n = 415).
Baseline Clinical Characteristics
Of the 415 participants, 62.65% did not receive adequate follow-up for the prevention of mother-to-child transmission (PMTCT) during their childhood and 47.23% of the children developed opportunistic infections (Table 2).
TABLE 2
| Variable (sampled = 415) | Category of variables | Frequency | Percent |
|---|---|---|---|
| PMCT | Yes | 155 | 37.35 |
| No | 260 | 62.65 | |
| OI at baseline rather than TB | Yes | 196 | 47.23 |
| No | 219 | 52.77 | |
| History of past TB Treatment |
YES | 52 | 12.53 |
| No | 363 | 87.47 | |
| WHO clinical stage at Baseline | Mild/stages 1 and 2 | 274 | 66.02 |
| advanced/stages 3 and 4 | 141 | 33.98 | |
| CD4 count at baseline | below threshold | 199 | 47.95 |
| Above threshold | 216 | 52.05 | |
| past OI prophylaxis | Yes | 274 | 66.02 |
| No | 141 | 33.98 | |
| baseline Hemoglobin (gm/dL) | Anemic | 85 | 20.48 |
| Normal | 330 | 79.52 | |
| Stunting | HAZ < −2 | 205 | 49.4 |
| Normal | 210 | 50.6 | |
| Thinness | BAZ < −2 | 156 | 37.6 |
| Normal | 256 | 62.4 | |
| Viral load status at base line in copy’s | Low viral load | 395 | 95.18 |
| High viral load | 20 | 4.82 |
Baseline clinical characteristics of children on Anti-retroviral treatment in Bahir Dar City, Northwest Ethiopia, 2021 (n = 415).
Follow-Up and Treatment-Related Characteristics
During the follow-up period, 67.23% and 15.68% of the participants demonstrated good and fair treatment adherence, respectively (Table 3). The majority of children (35.03%) developed bacterial pneumonia followed by diarrhea (33.99%) (Figure 1). The most common OI developed during follow-up was bacterial pneumonia. The most common regimens prescribed at ART initiation in the cohort were 4c = AZT-3TC-NVP 147 (35.42%), followed by 4a = d4t-3TC-EFV 122 (29.4%) (Figure 2).
TABLE 3
| Variable (sampled = 415) | Category of variables | Frequency | Percent |
|---|---|---|---|
| INH/TB prophylaxis | Taken | 283 | 68.19 |
| Missed | 132 | 31.81 | |
| Cotrimoxazole prophylaxis | Taken | 322 | 77.6 |
| Missed | 93 | 22.4 | |
| Adherence at follow-up | Good | 279 | 67.23 |
| Fair | 65 | 15.66 | |
| Poor | 71 | 17.11 | |
| treatment failure at follow-up | Yes | 113 | 27.23 |
| No | 302 | 72.77 | |
| vaccination status completed |
Complete | 240 | 57.83 |
| Incomplete | 175 | 42.17 | |
| TB contact history | Yes | 61 | 14.70 |
| No | 354 | 85.3 | |
| OI development at during follow up | Yes | 114 | 27.47 |
| No | 301 | 72.53 | |
| time from HIV positive confirmation to ART initiation | Timely | 201 | 48.43 |
| lately | 214 | 51.57 |
Follow-up clinical, immunological, laboratory and treatment-related characteristics of children on antiretroviral therapy between January 2011 and December 2020 in Bahir Dar city, Northwest Ethiopia, 2021.
FIGURE 1
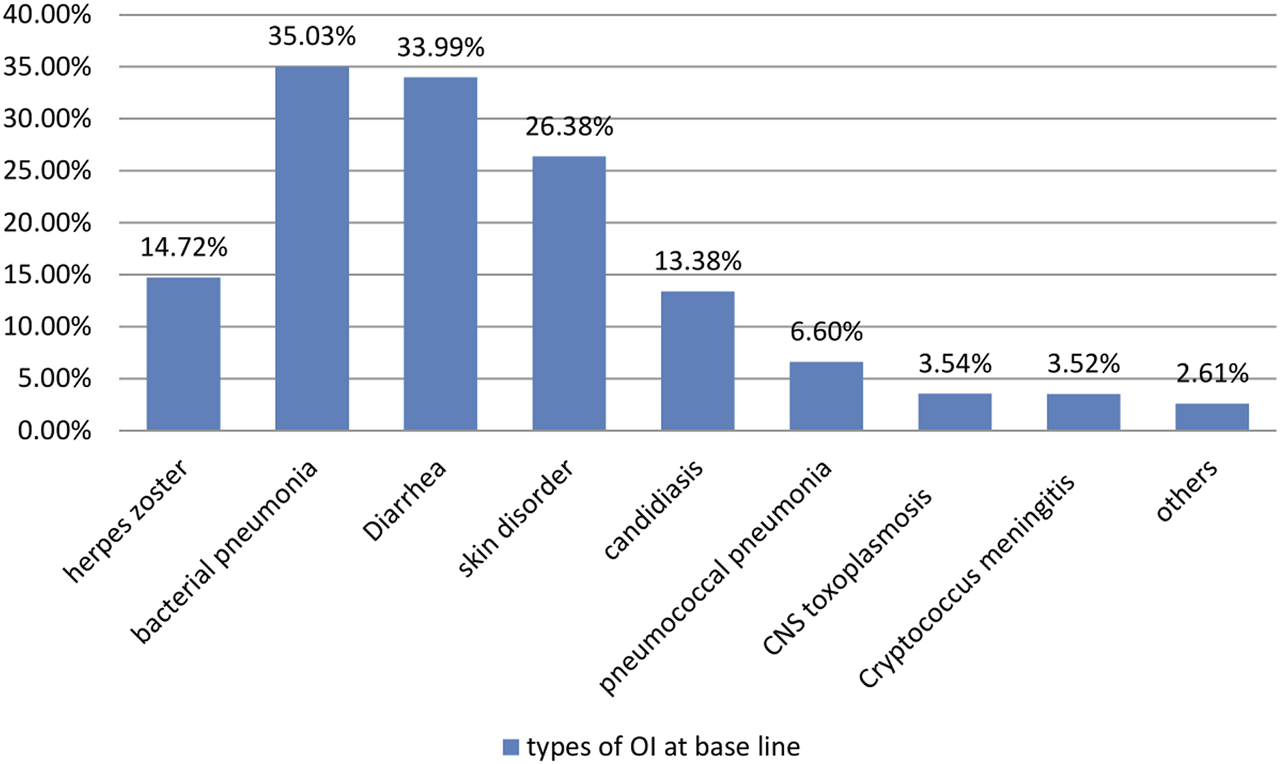
Opportunistic infections at baseline time of children on Anti-retroviral treatment started time at between January 2011 and December 2020 in Bahir Dar City, North West Ethiopia, 2021.
FIGURE 2
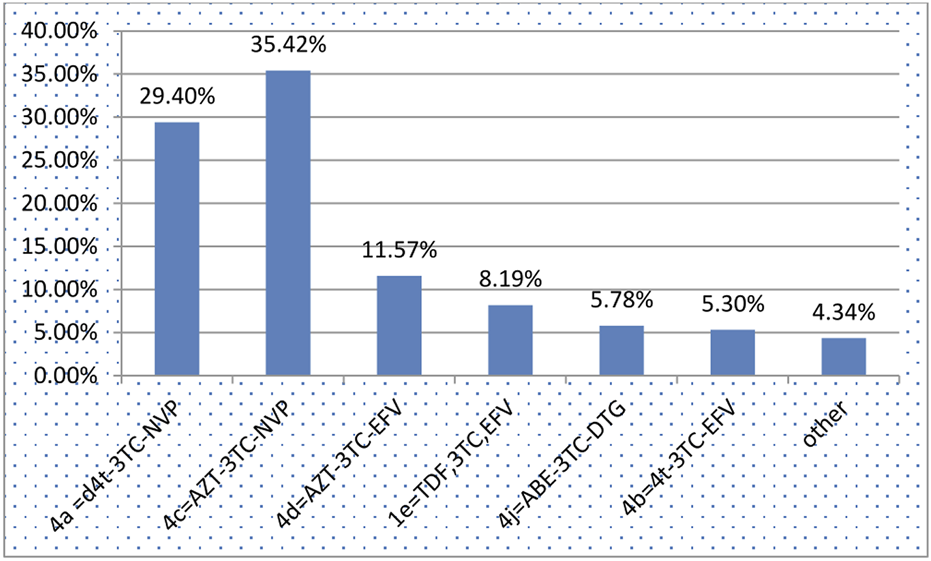
Anti-retroviral treatment regimens for children on Anti-retroviral treatment started time between January 2011 and December 2020 in Bahir Dar City, Northwest Ethiopia, 2021.
Time to Tuberculosis Development
A total of 415 children on ART were followed retrospectively in this study between January 2011 and December 2020, for a total of 1782.94 person-years of observation. Thus, 14.46% of individuals developed a new TB infection, resulting in an overall incidence density (ID) of TB development among children of 3.37 (95% CI: (2.612; 4.334) cases per 100 person-years. The median follow-up time was 49 months (IQR = 21.8–73.6), with a minimum of 3 months and a maximum of 120 months. The cumulative probability of tuberculosis-free survival at the end of the study was determined to be 76.5% (95% CI: 69.19%–81.97%).
At the end of the study, 68% of all participants were in follow-up or alive, 20% were transferred to other institutions, 8% were lost to follow-up, and 4% had died. Within 1 year of starting ART, 35% of new TB cases occurred, of which 71.67% were PTB and the remaining 8.33% were EPTB.
The overall Kaplan -Meier survival function showed that there was no median survival time for Tuberculosis development. The majority of TB cases occurred in the first year of ART initiation and decreased during follow-up, continuing steadily in later months of follow-up (Supplementary Figure S1).
Predictors of Tuberculosis Incidence
In bivariable Cox proportional regression analysis, children’s vaccination status, INH prophylaxis, cotrimoxazole prophylaxis, adherence to drug status, history of treatment failure, a history of contact with active TB patients, hemoglobin status, parental status, caregiver type, Stage of HIV/AIDS according to WHO clinical staging system, CD4 count, history of prophylaxis at baseline, height for age/HAZ and BAZ of children at baseline, viral load and treatment history for TB were variables with a p-value of <0.25 that were candidates for the final model. Then ART adherence status, INH prophylaxis, vaccination status, contact with active TB patients, and Stage of HIV/AIDS according to WHO clinical staging system were variables significantly associated with time to TB development at a p-value <0.05 in the final multivariable Cox proportional regression analysis model.
Children who did not receive Isoniazid preventive therapy were 2.55 times more likely to develop TB than children who received INH (AHR = 2.55: 95% CI, 1.12–5.81). When compared to the fully vaccinated children, HIV-infected children who had received incomplete vaccination had a 2.88-fold (AHR 2.88: 95% CI (1.14–7.28) higher risk of developing TB at any time. The risk of developing TB in children on ART with poor adherence was 3.48 times higher than in children with good adherence (AHR = 3.48; 95% CI: 1.38, 8–80). Furthermore, the risk of developing TB was 3.64 times higher in children on ART who had been exposed to active TB patients (AHR = 3.64; 95% CI (1.73–7.67) compared to children who had not been in contact with active TB patients Finally, Children on ART with advanced baseline WHO clinical stages (3 and 4) had a nearly two-fold higher risk (AHR: 2.18, 95% CI: 1.05–4.53) of developing TB than those with mild stages (1 and 2) (Table 4).
TABLE 4
| Variables | Category of variables | Outcome status | CHR [95%CI] | AHR [95%CI] | P-value | |
|---|---|---|---|---|---|---|
| Event | Censored | |||||
| Vaccination status | Completed | 9 | 231 | 1 | 1 | |
| Incomplete | 51 | 124 | 9.52 (4.69–19.35) | 2.89 (1.89–7.28) | 0.025** | |
| INH prophylaxis | Taken | 12 | 271 | 1 | 1 | |
| Missed | 48 | 84 | 13.18 (6.99–24.94) | 2.55 (1.12–5.81) | 0.026** | |
| Adherence | Good | 9 | 270 | 1 | 1 | |
| Fair | 13 | 52 | 6.76 (7.9–15.83) | 2.19 (0.8–5.99) | 0.13* | |
| Poor | 18 | 33 | 27.46 (13.2119.35) | 3.49 (1.38–8.8) | 0.008** | |
| Treatment failure | No | 12 | 290 | 1 | 1 | |
| Yes | 48 | 65 | 9.7 (5.16–18.3) | 1.28 (0.56–2.95) | 0.56* | |
| Contact history with TB patients | No | 18 | 20 | 1 | 1 | |
| Yes | 42 | 335 | 16.94 (9.66–29.7) | 3.64 (1.73–7.67) | 0.001** | |
| baseline Hgb status | Non-anemic | 17 | 313 | 1 | 1 | |
| Anemic | 43 | 42 | 12.4 (7.07–21.81) | 1.15 (0.48–2.75) | 0.758* | |
| Parental status | Both alive | 18 | 171 | 1 | 1 | |
| Mother/father dead | 24 | 131 | 1.73 (0.99–3.19) | 1.09 (0.5–2.34) | 0.83* | |
| Both dead | 18 | 53 | 3.17 (1.65–6.11) | 0.81 (0.36–1.8) | 0.6 | |
| Thinness | Normal | 31 | 228 | 1 | 1 | |
| Thinness (BAZ < -2) | 29 | 127 | 1.53 (0.92–2.54) | 0.95 (0.51–1.78) | 0.89* | |
| Stunting | Normal | 19 | 191 | 1 | 1 | |
| (HAZ < −2) | 41 | 164 | 2.78 (1.61–4.80) | 1.56 (0.82–2.93) | 0.17* | |
| WHO stage | Mild stage/1 and 2 | 12 | 262 | 1 | 1 | |
| Advanced stage/3 and 4 | 48 | 93 | 7.84 (4.16–14.77) | 2.18 (1.05–4.53) | 0.038** | |
| viral load | Low viral load | 52 | 343 | 1 | 1 | |
| High viral load | 8 | 40 | 3.08 (1.46–6.48) | 1.24 (0.24–3.13) | 0.65* | |
| baseline history of TB treatment | No | 18 | 336 | 1 | 1 | |
| Yes | 42 | 19 | 16.93 (9.66–29.7) | 1.35 (0.65–2.82) | 0.41* | |
| Cotrimoxazole | Taken | 22 | 300 | 1 | 1 | |
| Missed | 38 | 55 | 8.18 (4.83–13.86) | 1.18 (0.6–2.33) | 0.62* | |
| History of OI prophylaxis | Yes | 21 | 147 | 1 | 1 | |
| No | 39 | 102 | 5.13 (3.01–8.75) | 1.81 (0.92–3.56) | 0.08* | |
| Caregiver | Parent | 36 | 299 | 1 | 1 | |
| Sibling | 13 | 28 | 3.62 (1.78–6.34) | 1.42 (0.64–3.18) | 0.38* | |
| Grandparent | 6 | 26 | 2.66 (1.12–6.34) | 1.62 (0.57–4.60) | 0.36* | |
| orphan center | 5 | 8 | 4.8 (1.88–12.29) | 2.75 (0.87–8.68) | 0.08* | |
| CD4 count | Above threshold | 8 | 208 | 1 | 1 | |
| Below threshold | 52 | 147 | 7.95 (3.77–16.73) | 1.31 (0.53–3.24) | 0.55* | |
Bi-variable and Multi-variable Cox regression analysis model of predictors of developing tuberculosis among children on anti-retro-viral therapy between January 2011 and December 2020 in Bahir Dar city, North West Ethiopia, 2021.
1 = Reference category, * = p < 0.25, ** = p < 0.05.
Discussion
This study revealed that the overall incidence density was 3.37 (95% CI: 2.61; 4.33) cases per 100 PYs of observation. The current result was lower than previous studies conducted in other parts of Ethiopia such as the Beshangul region (9.6 per 100 PYs) [29] and Adama Referral Hospital and Medical College, and Oromia (6.03) per 100 child-years of observation [10]. However, it was higher than those of previous studies conducted in developed nations; the United Kingdom (0.196) per 100 PYs [32], and the United States of America (0.00302) per 100 PYs [33], and Latin America (0.28 per 100 PYs). This disparity may be attributed to the higher incidence of tuberculosis in resource-constrained settings. This suggests that additional efforts are still needed to reduce the incidence of TB, especially among children on ART.
At the end of the follow-up period, the cumulative survival probability of tuberculosis-free children on ART was 76.5% [95% CI: (69.63%; 82.02%)]. This is in line with the results of a study conducted at Gondar University, where the probability of TB-free cumulative survival was 76% [30].
Children with incomplete vaccination status had a higher risk of developing tuberculosis than fully vaccinated children. Previous studies conducted in Gondar [30], Beshangul Gumze, Ethiopia [34] Adama, Ethiopia [10], and Tanzania supported this finding [35]. This may be because BCG vaccination significantly reduces the risk of tuberculosis, particularly in children.
The study also discovered that adherence to ART drugs was a predictor of developing TB. Children with poor ART adherence had a higher risk of developing TB than those with good adherence. This finding was consistent with the results of a study conducted in Debre Markos, Amhara Region, Ethiopia [31]. The possible explanation would be that Poor adherence to ART results in a failure to suppress viral replication, increasing the likelihood of developing HIV mutations that could lead to the development of drug-resistant viral strains. Additionally, poor adherence to ART fails to prevent further viral destruction of the cellular immune system, resulting in a decline in CD4+ cell levels and the development of opportunistic infections.
This study demonstrated that advanced baseline WHO clinical stages 3 and 4 of HIV/AIDS were significant predictors of tuberculosis (TB) development in children on ART. This finding was similar with results conducted in UK [32] and Debre Markos, Ethiopia [31]. Deterioration of immunity in advanced WHO clinical stages accelerates the progression of latent TB infection to active TB infection, so children with advanced WHO clinical stages require close monitoring. Advanced HIV disease is associated with immunological deterioration, which leads to the activation of latent TB to active-stage TB. Furthermore, this study found that patients with a history of contact with TB patients were more likely to develop TB than children with no history of contact. Similar findings were reported in Ethiopia’s Beshangule Gumze regional state [29]. This may be due to the latent phase of tuberculosis being activated when immunocompromised children are exposed to active tuberculosis microbes. Finally, children who did not receive Isoniazid/INH prophylaxis had a higher risk of developing TB than their children who did. This finding is also consistent with a previous study conducted in northern Ethiopia [36], and it is supported by a study conducted in Ethiopia’s Adama Oromia regional state [10]. This could be because IPT reduces mycobacterium load and slows the progression of latent bacilli to active TB. In Ethiopia, where the prevalence of latent TB infection is high, the guideline for pediatric HIV/AIDS care and treatment recommends IPT for HIV-positive children who have been ruled out of active TB.
Limitations of the Study
This study did not address some important variables such as the impact of provider training, supplies, and equipment, on child survival due to the retrospective nature of the study. We will recommend to other researchers to conduct a prospective study in this field.
Conclusions and Recommendations
The incidence of tuberculosis among children on ART was high, particularly in the first year after ART was initiated. Contact history with an active TB patient, lack of Isoniazid preventive therapy, being at an advanced stage of WHO clinical stage, poor ART adherence, and incomplete vaccination status were risk factors for tuberculosis incidence. Hence, the existing TB/HIV prevention and control program should be strengthened to implement all packages that enable the reduction of the high incidence of tuberculosis among children on ART.
Statements
Ethics statement
The ethics committee of the Institutional Review Board (IRB) of Bahir-Dar University’s College of Medicine and Health Science approved this study. All methods were carried out in accordance with the National Research Ethics Review Guideline. This study followed the ethical standard of the Declaration of Helsinki. The need for informed consent was waived by the IRB of Bahir-Dar University based on our National Research Ethics Review Guideline which entitled “Waiver of informed consent or documentation of informed consent should be approved by the IRB.” By using this National Research Ethics Review Guideline statement on pages 38 and 39, the IRB committee declared no need for informed consent because of the nature of the study which used secondary data, and it was deemed unnecessary. In addition, waived informed consent by IRB was exercised due to the research project carrying no more than minimal risk according to the national regulation, and ethical approval guidelines.
Author contributions
GE conceived the study, wrote the proposal, and participated in the execution, acquisition of data, analysis, and interpretation of the results. MB, AK, and GW approved the proposal with extensive revisions, participated in the data analysis, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
First and foremost, we would like to thank Bahir Dar University for approving our title and publishing the manuscript in their Institutional Repository System (http://ir.bdu.edu.et/handle/123456789/15039). Second, we would like to thank the staff of all the ART centers, the card room, the administration, and the Amhara Public Health Institute for their willingness to allow us to collect the data. We would also like to thank the data collectors and supervisors for their efforts.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2025.1607892/full#supplementary-material
SUPPLEMENTARY FIGURE S1The Kaplan Meier overall survival curve with 95% confidence intervals among children on antiretroviral therapy between January 2011 and December 2020 in Bahir Dar City, Northwest Ethiopia, 2021.
References
1.
Lighter J Rigaud M . Diagnosing Childhood Tuberculosis: Traditional and Innovative Modalities. Curr Probl Pediatr Adolesc Health Care (2009) 39(3):61–88. 10.1016/j.cppeds.2008.12.003
2.
Nazar E Baghishani H Doosti H Ghavami V Aryan E Nasehi M et al Bayesian Spatial Survival Analysis of Duration to Cure Among New Smear-Positive Pulmonary Tuberculosis (PTB) Patients in Iran, during 2011-2018. Int J Environ Res Public Health (2020) 18(1):54. 10.3390/ijerph18010054
3.
Global H . AIDS Statistics—2018 Fact Sheet. Geneva: UNAIDS (2019).
4.
WHO. Roadmap towards Ending TB in Children and Adolescents (2018).
5.
CDC. HIV and Opportunistic Infections, Coinfections, and Conditions (2020).
6.
Abdela SG Diro E Zewdu FT Berhe FT Yeshaneh WE Tamirat KS et al Delayed Diagnosis and Ongoing Transmission of Leprosy in the Post-elimination Era in Boru Meda Hospital, Ethiopia. J Infect Dev Ctries (2020) 14:10S-15S. 10.3855/jidc.11706
7.
FMOH. Guidelines for Clinical and Programmatic Management of TB, TB/HIV and Leprosy in Ethiopia. In: Federal Democratic Republic of Ethiopia. Addis Ababa: Ministry of Health (2013).
8.
WHO. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children. Geneva, Switzerland: World Health Organization (2014).
9.
Buck WC Nguyen H Siapka M Basu L Cowan JG De Deus MI et al Integrated TB and HIV Care for Mozambican Children: Temporal Trends, Site-Level Determinants of Performance, and Recommendations for Improved TB Preventive Treatment. AIDS Res Ther (2021) 18(1):3–9. 10.1186/s12981-020-00325-9
10.
Beshir MT Beyene AH Tlaye KG Demelew TM . Incidence and Predictors of Tuberculosis Among HIV-Positive Children at Adama Referral Hospital and Medical College, Oromia, Ethiopia: A Retrospective Follow-Up Study. Epidemiol Health (2019) 41:e2019028. 10.4178/epih.e2019028
11.
WHO. Compendium of WHO Guidelines and Associated Standards: Ensuring Optimum Delivery of the Cascade of Care for Patients With Tuberculosis (2018).
12.
Howard AA El-Sadr WM . Integration of Tuberculosis and HIV Services in Sub-saharan Africa: Lessons Learned. Clin Infect Dis (2010) 50(Suppl. 3):S238–S44. 10.1086/651497
13.
Chakaya J Khan M Ntoumi F Aklillu E Fatima R Mwaba P et al Global Tuberculosis Report 2020 - Reflections on the Global TB Burden, Treatment and Prevention Efforts. Int J Infect Dis (2021) 113(Suppl. 1):S7–S12. 10.1016/j.ijid.2021.02.107
14.
Golub J Bur S Cronin W Gange S Baruch N Comstock G et al Delayed Tuberculosis Diagnosis and Tuberculosis Transmission. The Int J tuberculosis Lung Dis (2006) 10(1):24–30.
15.
Netherlands P . Global Strategy and Targets for Tuberculosis Prevention, Care and Control After (2015).
16.
Dodd PJ Yuen CM Sismanidis C Seddon JA Jenkins HE . The Global Burden of Tuberculosis Mortality in Children: A Mathematical Modelling Study. The Lancet Glob Health (2017) 5(9):e898–e906. 10.1016/S2214-109X(17)30289-9
17.
Tiberi S Migliori G-B Chakaya JM Kaesava T Al Abri SS Wejse C et al Cover Editorial-IJID World TB Day 2020 Theme Series. Elsevier (2020).
18.
WHO. WHO Consolidated Guidelines on Tuberculosis: Module 1: Prevention: Tuberculosis Preventive Treatment (2020).
19.
Golli A Niţu M Turcu F Popescu M Ciobanu-Mitrache L Olteanu M . Tuberculosis Remains a Public Health Problem in Romania. The Int J Tuberculosis Lung Dis (2019) 23(2):226–31. 10.5588/ijtld.18.0270
20.
Nigussie J Kasasa M Halefom G Hadush H Girma B . Predictors of Mortality Among Children Co-infected with Tuberculosis and Human Immunodeficiency Virus at General Hospitals of Two Zones of Tigray Region North Ethiopia, Retrospective Follow-Up Study. SSRN J (2021). 10.2139/ssrn.3765452
21.
Chopra K Arora V . End TB-Strategy-A Dream to Achieve. Indian J Tuberc (2019) 66(1):163–4. 10.1016/j.ijtb.2019.02.001
22.
Gelaw YA Assefa Y Soares Magalhaes RJ Demissie M Tadele W Dhewantara PW et al TB and HIV Epidemiology and Collaborative Service: Evidence From Ethiopia, 2011-2015. HIV AIDS (Auckl) (2020) 12:839–47.
23.
Purohit M Mustafa T . Laboratory Diagnosis of Extra-pulmonary Tuberculosis (EPTB) in Resource-Constrained Setting: State of the Art, Challenges and the Need. J Clin Diagn Res JCDR (2015) 9(4):EE01–6. 10.7860/JCDR/2015/12422.5792
24.
Jassal MS Bishai WR . Epidemiology and Challenges to the Elimination of Global Tuberculosis. Clin Infect Dis (2010) 50(Suppl. 3):S156–S64. 10.1086/651486
25.
Xu H Blair RV Veazey RS Wang X . Immunopathogenesis in HIV-Associated Pediatric Tuberculosis. Pediatr Res (2021) 91:21–6. 10.1038/s41390-021-01393-x
26.
Granich R Gupta S . 90-90-90 HIV Targets: Implications for HIV-Associated Tuberculosis. Curr Opin HIV AIDS (2018) 13(6):528–37. 10.1097/COH.0000000000000498
27.
Morishita F Viney K Lowbridge C Elsayed H Oh KH Rahevar K et al Epidemiology of Tuberculosis in the Western Pacific Region: Progress towards the 2020 Milestones of the End TB Strategy. West Pac Surveill response J WPSAR (2020) 11(4):10–23. 10.5365/wpsar.2020.11.3.002
28.
WHO. Global TB Report (2018). 15(1):1–11.
29.
kebede Bizuneh F Daba TT Mitiku BM shewano Fikretsion T . Time to Develop Tuberculosis and Predictors of Incidence Among Anti-retroviral Therapy Children on Two Selected Hospitals at Benishangule Gumuz Region, North West Ethiopia. A retrospective cohort study (2020).
30.
Ayalaw SG Alene KA Adane AA . Incidence and Predictors of Tuberculosis Among HIV Positive Children at University of Gondar Referral Hospital, Northwest Ethiopia: A Retrospective Follow-Up Study. Int scholarly Res notices (2015) 2015:307810. 10.1155/2015/307810
31.
Endalamaw A Engeda EH Tezera N . Incidence of Tuberculosis in Children on Antiretroviral Therapy: A Retrospective Cohort Study. BMC Res Notes (2018) 11(1):745–7. 10.1186/s13104-018-3846-z
32.
Turkova A Chappell E Judd A Goodall RL Welch SB Foster C et al Prevalence, Incidence, and Associated Risk Factors of Tuberculosis in Children With HIV Living in the UK and Ireland (CHIPS): A Cohort Study. The Lancet HIV (2015) 2(12):e530–e539. 10.1016/S2352-3018(15)00200-3
33.
Scott C Kirking HL Jeffries C Price SF Pratt R , Centers for Disease Control and Prevention CDC. Tuberculosis Trends — United States, 2014. Morbidity Mortality Weekly Rep (2015) 64(10):265–9.
34.
Boneya DJ Dessie AA . Time to Develop Pulmonary Tuberculosis and Predictors Among HIV Infected Children Receiving Anti-retroviral Therapy. In: Assosa and Pawe General Hospitals, North West Ethiopia 2020 (2020).
35.
Faurholt-Jepsen D Range N PrayGod G Jeremiah K Faurholt-Jepsen M Aabye MG et al BCG Protects Against Tuberculosis Irrespective of HIV Status: A Matched Case-Control Study in Mwanza, Tanzania. Thorax (2013) 68(3):288–9. 10.1136/thoraxjnl-2012-201971
36.
Alemu YM Andargie G Gebeye E . High Incidence of Tuberculosis in the Absence of Isoniazid and Cotrimoxazole Preventive Therapy in Children Living With HIV in Northern Ethiopia: A Retrospective Follow-Up Study. PLoS One (2016) 11(4):e0152941. 10.1371/journal.pone.0152941
Summary
Keywords
ART, child health, WHO clinical staging, incidence, infectious disease
Citation
Endalew G, Beyene MB, Kassie A and Wassie GT (2025) Incidence and Risk Factors of Tuberculosis among Children Receiving Antiretroviral Therapy in Northwest, Ethiopia. Int. J. Public Health 70:1607892. doi: 10.3389/ijph.2025.1607892
Received
23 August 2024
Accepted
27 February 2025
Published
20 March 2025
Volume
70 - 2025
Edited by
Tah Yves-Nathan Tian Bi, Félix Houphouët-Boigny University, Côte d’Ivoire
Reviewed by
Rufin Kouassi Assare, Félix Houphouët-Boigny University, Côte d’Ivoire
One reviewer who chose to remain anonymous
Updates
Copyright
© 2025 Endalew, Beyene, Kassie and Wassie.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gizachew Tadesse Wassie, leulgzat@gmail.com, gizachew.tadesse@bdu.edu.et
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.