Abstract
Objective:
To characterize the clinical phenotypes of SARS-CoV-2 infection in hospitalized children as part of the EPICO multicenter cohort study.
Methods:
We included hospitalized children with confirmed SARS-CoV-2 infection from Colombian and Spanish institutions to assess disease evolution and outcomes. Cluster analysis was performed to identify clinical phenotypes.
Results:
A total of 2318 patients were included (55% male, 36% infants). Five phenotype clusters emerged: Cluster 1 (26.5%): infants without comorbidities, low PICU admissions and mortality; Cluster 2 (18.5%): respiratory comorbidities, high microorganism co-detection and mortality; Cluster 3 (11.5%): fever, gastrointestinal symptoms, high PICU admissions; Cluster 4 (32%): mild unspecific symptoms, low mortality; Cluster 5 (11.3%): adolescents without comorbidities, low co-detection and hospitalization rates. Findings were consistent across both countries.
Conclusion:
Identifying clinical phenotypes of SARS-CoV-2 in children may improve risk stratification and guide future management strategies.
Introduction
Since the onset of the COVID-19 (acronym for Coronavirus Disease of 2019) [1] pandemic in 2020, there has been extensive documentation of the disease in adult populations and diagnostic approaches that focus on symptom-based screening in children [2]. However, there is a lack of information and evidence regarding the manifestations, diagnosis, treatment, and prognosis of COVID-19 in the pediatric population, which may be significant for acute decision-making and prognosis.
The behavior of the virus in pediatric patients has been characterized by constant changes and unpredictability. In general, children with COVID-19 have a lower risk of hospitalization and severe complications [3]. Various studies have reported that up to 17% of pediatric patients are asymptomatic, while approximately 63% exhibit mild symptoms [4], emphasizing that the percentage of asymptomatic patients may be underestimated, as most do not get tested for SARS-CoV-2 [3]. Besides, we already know that COVID-19 is causing major physical, psychological, developmental, behavioral, and social health consequences for children, having an impact even in mortality [5, 6].
In 2020, a severe disease phenotype related to children infected with SARS-CoV-2 was initially identified in South London (UK) [7]. Following this discovery, a prospective cohort study conducted in England, Wales, and Scotland [8] described three distinct clusters of clinical phenotypes: discrete respiratory illness cluster, systemic mucocutaneous enteric illness cluster, and neurological illness cluster. Later another cross-sectional multicenter study, nested within the cohort EPICO-AE (Epidemiological Study of COVID-19 in Children of the Spanish Society of Pediatrics), also identified three phenotypes: Lower Respiratory, Gastrointestinal and Flu-like [2]. These investigations utilized symptom-based cluster analysis to categorize the disease presentations.
While some studies had focused exclusively on the multisystem inflammatory syndrome in children (MIS-C) and described phenotypes associated with the immune response to SARS-CoV-2 phenotype [9, 10], they have overlooked other disease presentations. Other studies have also differentiated between MIS-C and acute forms of the disease; however, they do not distinguish between the different acute presentations beyond a description of the symptoms that may occur [11, 12]. Similarly, other studies have described specific manifestations, such as neurologic or ophthalmological complications of the disease in pediatric patients, without including all possible presentations as distinct phenotypes [13, 14].
Furthermore, although certain studies, have mentioned clinical presentation, treatment and outcomes [2, 8, 11, 15] these aspects were not incorporated together into a cluster analysis, and, importantly, there is a lack of information regarding the population of Latin America, with only observational and descriptive data in terms of prevalence but a lack of data in terms of clinical presentation [16]. This study aims to describe and define the disease phenotypes among hospitalized pediatric population aged 29 days to 17 years, affected by SARS-CoV-2 infection included in the EPICO cohort in Colombia and Spain, using cluster analysis.
Methods
Study Design and Settings
The EPICO (Epidemiological study of respiratory infections caused by SARS-CoV-2 infection in pediatric population) is a multicentric cohort study conducted to investigate the behavior and hospital spectrum of SARS-CoV-2 infection in the pediatric population, including its evolution, severity factors, and outcomes between April 2020 and November 2021. The cohort included 13 hospitals in Colombia and 55 hospitals in Spain. The Colombian hospitals were predominantly located in the capital, Bogotá D.C., while the Spanish hospitals were spread across 13 provinces. Figure 1 shows the flowchart of EPICO cohort, and Supplementary Material S1 includes additional information about the institutions included. More information about the study, can be found in [17].
FIGURE 1
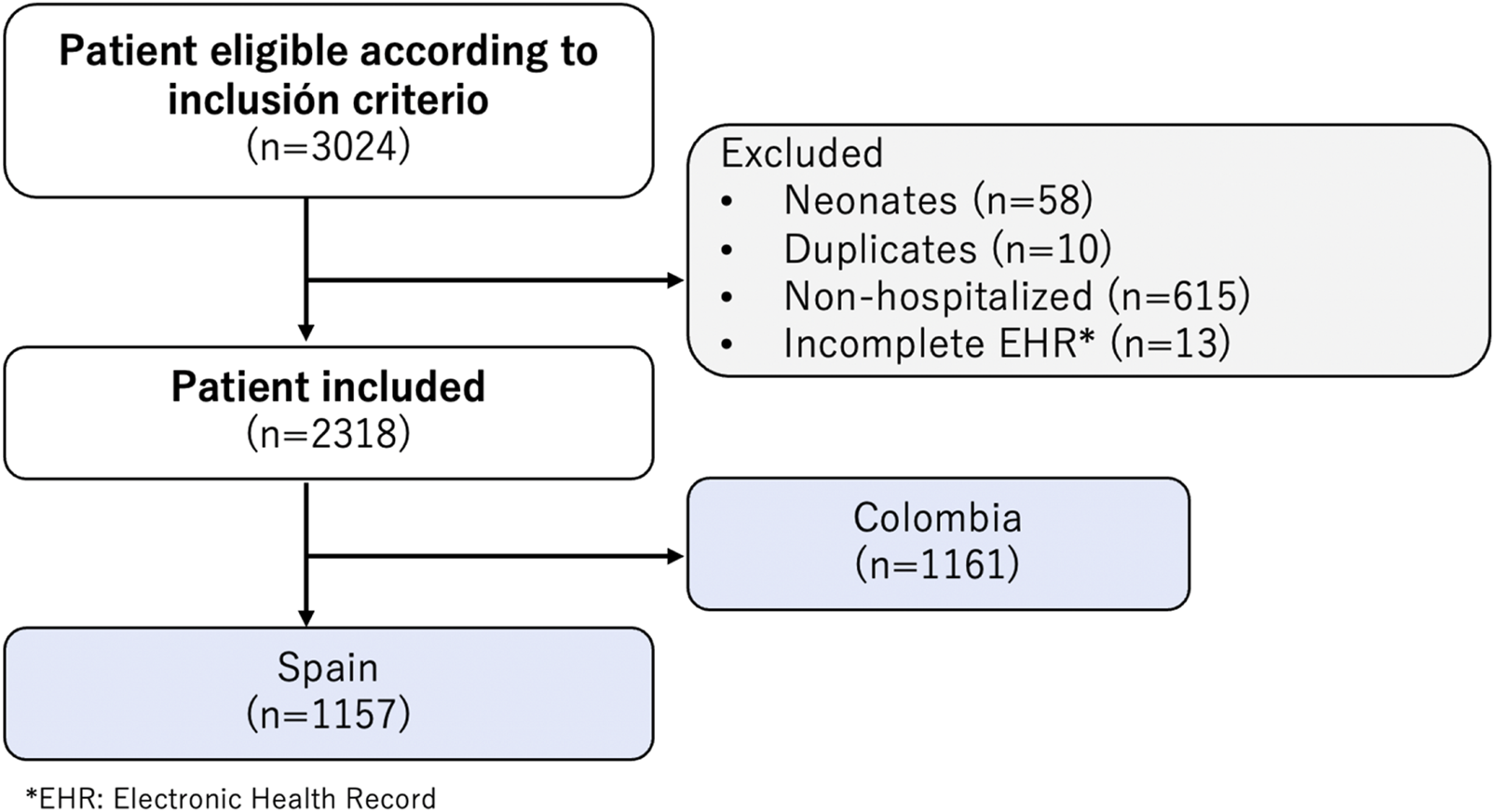
Flowchart of Epidemiological Study of COVID-19 in Children of the Spanish Society of Pediatrics (EPICO) cohort study and Institutions included (EPICO Study. Bogotá, Colombia, 2021).
Participants
Clinical records from eligible patients (children aged 29 days to 17 years, hospitalized with confirmed SARS-CoV-2 infection by either method [PCR (Protein Chain Reaction), antigen detection, or antibody testing Immunoglobulin G (IgG)/Immunoglobulin M (IgM +)] were included. The participants were admitted to the emergency room, inpatient ward, or pediatric critical care unit (PICU). Clinical records that met the inclusion criteria were selected by clinical research staff through daily review. Patients who were admitted to the emergency room and did not require hospital admission were excluded. We did not perform a sample size calculation because we had a significant amount of uncertainty regarding the epidemiology of the disease. The number of pediatric patients hospitalized in participating hospitals in Colombia and Spain with confirmed SARS-CoV-2 infection determined the sample size for this study. The patients were followed throughout their hospitalization, with no outpatient monitoring conducted.
Data Sources
The data corresponding to the variables selected for the study were recorded retrospectively from clinical records and standardized in a digital format (REDCap), which only the researchers had access to maintain confidentiality. The development of COVID-19 infection was analyzed as the primary outcome, and five main categories were considered for the collection of variables: demographic characteristics, comorbidities, signs and symptoms upon admission, laboratory variables, and variables related to patients’ evolution and outcomes (treatment, medication, complications, hospital stay, and ventilation).
Variables and Categorizing Variables
A stepwise protocol was developed to select and organize variables for cluster analysis. All variables were collected from primary sources within the participating institutions.
A total of 255 variables were collected and classified into three main categories according to the literature review: demographic, clinical, and complementary variables. Subsequently, the research group selected 25 variables through clinical experience and an individual literature review, followed by a meeting and discussion to ensure no variables of potential clinical value were overlooked when classifying the sample. The three factors considered for filtering the variables were:
- Clinical manifestations, management, and outcomes described and observed for COVID-19 and other acute respiratory diseases based on age.
- Severity of acute disease according to microbiological codetection and characteristics of the microorganisms involved, as seen in other diseases such as acute bronchitis [18–20].
- Correlation between the severity of the disease and the patient’s outcomes with their comorbidities, a finding that is notable in COVID-19 among adults [4, 21–23].
Data Analysis
We used descriptive statistics for all variables of interest. For qualitative variables (all variables except length of hospitalization), we used absolute and relative frequencies. Quantitative variables were expressed by measures of central tendency and dispersion (appropriate according to their distribution).
To classify the different clinical phenotypes of the presentation of COVID-19 in pediatric patients, a cluster analysis was performed based on an agglomerative hierarchical ward analysis. This technique is a type of hierarchical clustering method that builds nested clusters by repeatedly merging pairs of clusters that minimize the increase in the overall within-cluster variance. Specifically, Ward’s method focuses on minimizing the sum of squared differences within clusters, making it particularly effective for creating clusters with relatively similar sizes [24]. In this analysis, categorical and continuous variables can be used, but in our study, we focused exclusively on qualitative variables to assess the clinical phenotypes. The Gower coefficient, which is well-suited for mixed data types, was used to calculate the similarity between patients. This method does not require large sample sizes, but the number of clusters can be influenced by the number of variables and the underlying distribution of the data. Ward’s method is advantageous over other clustering methods due to its ability to minimized within-cluster variance; however, it can be sensitive to outliers and may requiere careful interpretation to avoid overfitting.
The optical number of clusters was initially determined by visually inspecting the dendrogram, a tree-like diagram that represents the order in which clusters are merged. This was further confirmed by using the Elbow method, which assesses the percentage of variance explaned as a function of the number of clusters, and the Silhouette coefficient, which measures how similar each observation is to its own cluster compared to other clusters [25]. To perform the comparative analysis between the clusters and the outcomes of interest, we used ANOVA, X2, or Fisher’s exact tests, Student’s t-test, or the Mann-Whitney test, depending on the data type and distribution, with a significance level set at 5%.
All statistical analyzes were performed in R (version 4.0.4). The Colombian and Spanish cohort databases were combined by including only patients hospitalized between April 2020 and November 2021. EPICO Spain and EPICO Colombia used the same collection instrument to ensure comparability between the two cohorts.
For more detailed information on the agglomerative hierarchical ward’s method, please refer to key publications such as Murtagh and Legendre [26] on hierarchical clustering algorithms, and Milligan [27] for a detailed comparison of Ward´s method with other clustering techniques.
Results
A total of 2,318 patients were included in the study, with equal distribution from the Colombian and Spanish institutions (50% vs. 50% respectively, 55% were male). The age distribution showed that infants accounted for the highest proportion (36%), followed by adolescents (23%), preschoolers (21%) and school-aged children (20%). Most patients (71%) did not report any comorbidity, while 18% had a non-respiratory comorbidity, and the remaining patients reported any type of respiratory comorbidity. During diagnosis fever was the main symptom (71%), followed by cough (48%), rhinorrhea (45%), vomiting and nausea (26%), abdominal pain (21%), as the most common findings. As for treatment, 655 (27%) patients required steroid therapy, while 862 (36%) completed antibiotic treatment. Additional details are provided in Table 1.
TABLE 1
| Variable | N = 23,181 |
|---|---|
| Country | |
| Colombia | 1,161 (50%) |
| Spain | 1,157 (50%) |
| Age group | |
| Infant | 826 (36%) |
| Preschooler | 497 (21%) |
| School-aged children | 470 (20%) |
| Adolescents | 525 (23%) |
| Sex | |
| Male | 1,268 (55%) |
| Female | 1,049 (45%) |
| Non specified | 1 (<0.1%) |
| Codetection | 313 (14%) |
| Comorbidities | |
| None | 1,484 (64%) |
| Non-respiratory comorbidity | 490 (21%) |
| Respiratory comorbidity | 344 (15%) |
| Treatment | |
| Steroid treatment | 730 (31%) |
| Antiobiotic treatment | 985 (42%) |
| Symptoms | |
| Fever | 1,681 (73%) |
| Cough | 1,130 (49%) |
| Rhinorrhea | 1,016 (44%) |
| Wheezing | 373 (16%) |
| Altered consciousness/confusion | 120 (5.2%) |
| Abdominal pain | 556 (24%) |
| Vomiting/Nausea | 650 (28%) |
| Diarrhea | 543 (23%) |
| Pale skin | 133 (5.7%) |
| Acne | 212 (9.1%) |
| Lymphadenopathy | 77 (3.3%) |
| Capillary refil time > 2 s | 79 (3.4%) |
| Shock | 140 (6.0%) |
| 1 n (%) | |
General characteristics of Epidemiological Study of COVID-19 in Children of the Spanish Society of Pediatrics (EPICO) cohort (EPICO Study. Bogotá, Colombia, 2021).
The cluster analysis resulted in the identification of five distinct phenotype clusters (Figure 2). The determination of the number of clusters was based on the results from the dendrogram (Supplementary Material S4), where the cut-off point was chosen after observing the largest jump in dissimilarity, indicating the optimal number of clusters. This was further validated using the Elbow method, which showed a clear inflection point at five clusters. The Silhouette coefficient confirmed that this model provided the best balance between cohesion and separation among clusters, with a coefficient value indicating good overall clustering quality. Several potential models were initially identified; however, the selected five-cluster model was chosen for its clinical relevance and interpretability. This model effectively captured the diversity in clinical phenotypes while maintaining statistical robustness.
FIGURE 2
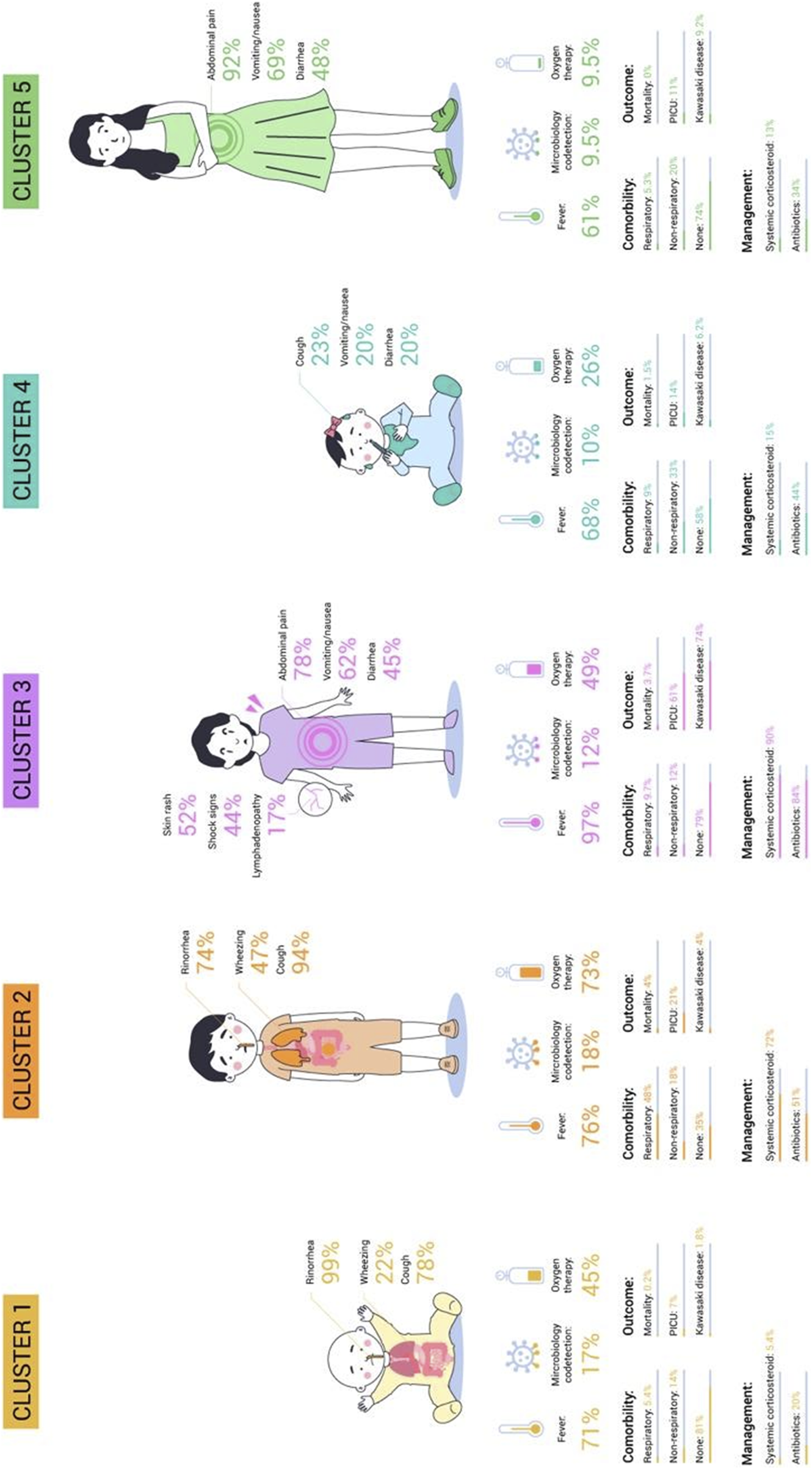
Clinical Phenotypes in Epidemiological Study of COVID-19 in Children of the Spanish Society of Pediatrics (EPICO) Cohort study (EPICO Study. Bogotá, Colombia, 2021).
All clinical-epidemiologic characteristics were found to significantly differ between the groups (p-value <0.005). Cluster 4 was the most common (n = 744, 32%), while cluster 5 was the least common (n = 262, 11.3%). The specifics of each cluster are described individually below:
Cluster 1 (26.57%) predominantly consisted of infants without comorbidities. The primary symptoms observed in Cluster 1 were rhinorrhea (99%) and cough (78%), which are characteristic of upper respiratory tract symptoms, without wheezing. Patients in this cluster were treated mostly with oxygen therapy (45%) and had the lowest rates of admission to the PICU (7%) and mortality (0.2%).
Cluster 2 (18.5%) showed no significant age differences and was mostly male patients with respiratory comorbidities. The primary symptoms observed were cough (94%) and wheezing (47%), indicating a predominance of lower respiratory tract symptoms. Seventy-two percent of patients required systemic corticosteroids, 51% received antibiotic treatment. This cluster had the highest microbiological codetection rates (18%), need for oxygen therapy (73%), and morality rates (4%).
Cluster 3 (11.51%) was frequent in male patients without comorbidities. The most common symptoms were fever (97%) and gastrointestinal symptoms including abdominal pain (78%), vomiting/nausea (62%) and diarrhea (45%). Cutaneous rash (52%), shock signs (44%) and lymphadenopathy (17%) were also observed. This cluster had the greatest use of antibiotics (84%), systemic corticosteroids (90%) as well as the highest admission rate to the PICU (61%) and complications related to suspected Kawasaki like diseases (74%). The mortality rate in this cluster was 3.7%.
Cluster 4 (32.09%) showed no significant differences in age or sex and was characterized by patients with non-respiratory comorbidities. It was also characterized by fever (68%) and mild and non-specific symptoms related to the digestive and respiratory systems. This cluster also received antibiotics (44%) and the mortality rate was low (1.5%).
Cluster 5 (11.3%) consisted primarily of adolescents (39%) and female patients (58%) without comorbidities. Gastrointestinal symptoms including abdominal pain (92%), vomiting/nausea (69%) and diarrhea (48%) were prominent in this cluster. This cluster had the lowest rate of microbiology co-detection (9.5%) and received antibiotics in 34% of cases. The admission to a pediatric intensive care unit (PICU) and hospitalization rates were the lowest (11% and 3.7%, respectively). This cohort had no mortality.
In both countries, the five cluster groups were generally comparable, with few distinctions between them. Clusters 1 and 2 consisted primarily of Colombian patients (61% and 59% respectively), while cluster 3 was predominantly composed of Spanish patients (75%). Notably, there were disparities in mortality rates, with cluster 2 in Spain and cluster 3 in Colombia having the greatest mortality rates (Table 2).
TABLE 2
| Variable | N | 1, N = 616a | 2, N = 429a | 3, N = 267a | 4, N = 744a | 5, N = 262a | p-valueb |
|---|---|---|---|---|---|---|---|
| Country | 2.318 | <0.001 | |||||
| Colombia | 374 (61%) | 254 (59%) | 66 (25%) | 339 (46%) | 128 (49%) | ||
| Spain | 242 (39%) | 175 (41%) | 201 (75%) | 405 (54%) | 134 (51%) | ||
| Age group | 2.318 | <0.001 | |||||
| Infant | 402 (65%) | 112 (26%) | 18 (6.7%) | 266 (36%) | 28 (11%) | ||
| Preschooler | 108 (18%) | 141 (33%) | 46 (17%) | 157 (21%) | 45 (17%) | ||
| School aged | 44 (7.1%) | 96 (22%) | 113 (42%) | 129 (17%) | 88 (34%) | ||
| Adolescent | 62 (10%) | 80 (19%) | 90 (34%) | 192 (26%) | 101 (39%) | ||
| Sex | 2.318 | ||||||
| Male | 318 (52%) | 277 (65%) | 180 (67%) | 383 (51%) | 110 (42%) | ||
| Female | 298 (48%) | 152 (35%) | 87 (33%) | 360 (48%) | 152 (58%) | ||
| Not specified | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.1%) | 0 (0%) | ||
| Codetection | 2.318 | 103 (17%) | 76 (18%) | 31 (12%) | 78 (10%) | 25 (9.5%) | <0.001 |
| Comorbidity status | 2.318 | <0.001 | |||||
| No comorbidities | 496 (81%) | 149 (35%) | 210 (79%) | 434 (58%) | 195 (74%) | ||
| Non respiratory comorbidity | 87 (14%) | 76 (18%) | 31 (12%) | 243 (33%) | 53 (20%) | ||
| Respiratory comorbidity | 33 (5.4%) | 204 (48%) | 26 (9.7%) | 67 (9.0%) | 14 (5.3%) | ||
| Treatment | |||||||
| Systemic corticosteroids | 2.318 | 33 (5.4%) | 309 (72%) | 241 (90%) | 113 (15%) | 34 (13%) | <0.001 |
| Antibiotic | 2.318 | 126 (20%) | 219 (51%) | 225 (84%) | 327 (44%) | 88 (34%) | <0.001 |
| Symptoms | |||||||
| History of fever | 2.318 | 438 (71%) | 324 (76%) | 255 (96%) | 505 (68%) | 159 (61%) | <0.001 |
| Cough | 2.318 | 480 (78%) | 404 (94%) | 49 (18%) | 173 (23%) | 24 (9.2%) | <0.001 |
| Rhinorrhea | 2.318 | 607 (99%) | 317 (74%) | 33 (12%) | 39 (5.2%) | 20 (7.6%) | <0.001 |
| Wheezing | 2.318 | 138 (22%) | 203 (47%) | 9 (3.4%) | 22 (3.0%) | 1 (0.4%) | <0.001 |
| Altered consciousness/confusion | 2.318 | 17 (2.8%) | 18 (4.2%) | 44 (16%) | 37 (5.0%) | 4 (1.5%) | <0.001 |
| Abdominal pain | 2.318 | 47 (7.6%) | 20 (4.7%) | 208 (78%) | 41 (5.5%) | 240 (92%) | <0.001 |
| Vomiting/Nausea | 2.318 | 101 (16%) | 52 (12%) | 166 (62%) | 149 (20%) | 182 (69%) | <0.001 |
| Diarrhoea | 2.318 | 115 (19%) | 36 (8.4%) | 120 (45%) | 146 (20%) | 126 (48%) | <0.001 |
| Pale/mottled skin | 2.318 | 25 (4.1%) | 16 (3.7%) | 53 (20%) | 33 (4.4%) | 6 (2.3%) | <0.001 |
| Skin rash | 2.318 | 9 (1.5%) | 5 (1.2%) | 138 (52%) | 56 (7.5%) | 4 (1.5%) | <0.001 |
| Lymphadenopathy | 2.318 | 4 (0.6%) | 3 (0.7%) | 46 (17%) | 23 (3.1%) | 1 (0.4%) | <0.001 |
| Capillary refill time >2 s? | 2.318 | 4 (0.6%) | 7 (1.6%) | 59 (22%) | 5 (0.7%) | 4 (1.5%) | <0.001 |
| Shock signs | 2.318 | 3 (0.5%) | 6 (1.4%) | 118 (44%) | 12 (1.6%) | 1 (0.4%) | <0.001 |
| Clinical outcomes | |||||||
| Hospital time in days, median (IQR) | 2.287 | 4 (3.6) | 5 (4.9) | 9 (6.12) | 5 (3.8) | 4 (3.7) | <0.001 |
| Admission to PICU (yes)c | 2.318 | 43 (7.0%) | 91 (21%) | 163 (61%) | 107 (14%) | 28 (11%) | <0.001 |
| Oxygen therapyc | 2.318 | 276 (45%) | 312 (73%) | 131 (49%) | 196 (26%) | 25 (9.5%) | <0.001 |
| Kawasaki complication | 2.318 | 11 (1.8%) | 17 (4.0%) | 197 (74%) | 46 (6.2%) | 24 (9.2%) | <0.001 |
| Death | 2.318 | 1 (0.2%) | 17 (4.0%) | 10 (3.7%) | 11 (1.5%) | 0 (0%) | <0.001 |
Total Phenotypes of COVID-19 presentation in hospitalized pediatric patients in Colombia and Spain (EPICO Study. Bogotá, Colombia, 2021).
n (%); Median (IQR).
Pearson’s Chi-squared tests; Kruskal-Wallis rank sum test; Fisher’s exact test.
Missing values categorized as “No;” Pearson’s Chi-squared test; Kruskal-Wallis rank sum test; Fisher’s exact test.
In terms of clinical characteristics, the predominance of cough, fever, shortness of breath, nasal discharge and wheezing as the most frequent symptoms observed among the patients. For more details on each of the clusters by country please refer to the Supplementary Material S2, S3.
Discussion
This study presents the findings of the only large-scale study conducted in Spanish-speaking hospitals, providing insights into the phenotypes of COVID-19 disease in infants and adolescents. Five distinct clinical phenotypes of COVID-19 disease were identified. Cluster 1 (26.57%) consisted of infants without comorbidities, with low PICU admission and mortality rates. Cluster 2 (18.5%) had respiratory comorbidities, high co-detection, and mortality rates. Cluster 3 (11.51%) showed fever, gastrointestinal symptoms, and high PICU admission. Cluster 4 (32.09%) had mild unspecific symptoms and low mortality. Cluster 5 (11.3%) included adolescents without comorbidities, with low co-detection and hospitalization rates. Comparable findings were observed in both countries.
Swann et al. described three clusters of clinical phenotypes: discrete respiratory illness, systemic mucocutaneous enteric illness, and neurological clusters [8]. Similarly, Cobos-Carrascosa et al. identified three phenotypes: lower respiratory, gastrointestinal, and flu-like, based on the EPICO-AE cohort [2]. However, it is important to note that these previous studies focused primarily on describing the presentation of the disease and did not provide a comprehensive description of variables such as treatment and outcomes. In contrast, our study not only categorized the different phenotypes but also incorporated treatment and outcome variables, which are crucial for guiding clinical management and prognosis.
In our study, cluster 1 was characterized by moderate upper respiratory symptoms correlating with previously published data on COVID-19 in the pediatric population, indicating that most pediatric patients are asymptomatic or have mild respiratory disease [3, 28–30]. Cluster 2 was characterized by lower respiratory symptoms with fever, cough, and wheezing as predominant symptoms, which is consistent with the reported in literature (26. The increased incidence of respiratory comorbidities and higher mortality rates can explain the more severe presentation of respiratory disease in Cluster 2 compared to Cluster 1. This is consistent with the findings of respiratory comorbidities, cardiac, cardiorespiratory and gastrointestinal complications as significant risk factors for the disease´s severe complications and admission to the Pediatric Intensive Care Unit (PICU) [31, 32].
Most patients in Cluster 3 display clinical characteristics consistent with MIS-C (Multisystem Inflammatory Syndrome in Children), a severe form of COVID-19 presentation in response to an intensive inflammatory response. This syndrome has been thoroughly described in the medical literature [9] and has also been observed in infections caused by other viruses, such as adenovirus [33, 34].
In Cluster 4, fever was the primary symptom of the disease, along with other non-specific symptoms. This could be explained by the fact that individuals in this cohort were tested for SARS-CoV-2 as part of routine evaluations in which COVID-19 was not the primary diagnosis. Instead, SARS-CoV-2 infection was discovered by accident. Cluster 5 was characterized by gastrointestinal symptoms and included predominantly female adolescents. Diarrhea has been reported as a significant symptom in COVID-19 infected patients. According to a study by Poeta M. et al., the global prevalence of this symptom is 18.9%, with a higher prevalence in children aged 5–11 years [35].
Other studies comparing clinical characteristics in children with SARS-CoV-2 infection identified manifestations such as myalgia, headache or dysgeusia but generally with low sensitivity [36–38]. All the differences between these studies and ours could be explained by the inclusion of hospitalized patients.
One strength of the study is the sample size, which includes two pediatric populations with different healthcare systems, cultural and socio-demographic characteristics. Similarly, it was conducted during a significant period of the pandemic, encompassing 2 years of data collection that captured both peaks and valleys of the disease. Furthermore, information from all age groups is included.
On the other hand, this study has some limitations, predominantly attributable to the selection of patient cohort from institutions that participate. Therefore, the information was retrieved retrospectively from the patient’s medical records without any active data collection. In addition, the participating institutions lacked a standardized protocol for disease management.
The identification of distinct clinical phenotypes in our study offers significant potential to improve the management of pediatric COVID-19. Recognizing the various ways in which SARS-CoV-2 manifests in children enables more precise treatment strategies. For instance, understanding that infants without comorbidities generally have low PICU admission and mortality rates (Cluster 1) can help clinicians avoid unnecessary interventions. Conversely, identifying high-risk groups, such as those with respiratory comorbidities and higher mortality rates (Cluster 2), emphasizes the importance of vigilant monitoring and early intervention.
These findings provide a foundation for developing personalized clinical guidelines that enhance risk stratification and resource allocation. This could involve creating in the future a risk classification system tailored to the pediatric population with SARS-CoV-2, incorporating factors like age, comorbidities, and clinical presentation. Such an approach would improve personalized care and optimize hospital resource allocation, ultimately leading to better patient outcomes.
Conclusion
Our study successfully identified five phenotypic patterns of SARS-CoV-2 infection in hospitalized pediatric population, encompassing clinical manifestations, management, and prognosis. This information could be utilized to implement differential approaches, classify risks, and define individualized management strategies for patients in this age group. Efforts should be focused on devising and implementing management guidelines, as well as preventing the spread of infectious diseases.
Statements
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics statement
The studies involving humans were approved by protocols CCEI-12686-2020, CEIFUS 1852-20, CEIFUS 1853-20, CEIFUS 1851-20, CEIFUS 1903-20, CEISH 0629-2020, SDM-034-20. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this was a retrospective study and we used electronic data record for the analysis.
Author contributions
AR, PV-H, MM-R, OB, LM, JP, SM-L, SR-G, and MV: contributed to the conceptualization and design of the study. MN, GF, and SM: organized the database and performed the statistical analysis. MS-A, MG, NB, LO, MD, MV, GF, LN-R, PV-H, SM, and AR: wrote the first draft to the manuscript. AD-D and SM: wrote sections of the manuscript. AD-D, CÁ-M, IG-T, AM, WB, CG, MA, JN, PP, CB-A, RV, IG, CM, and AT: contributed to the editing to the final paper. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was fund by the Pediatrics Department at the Universidad de los Andes and Fundación Santa Fe de Bogotá and Pan American Health Organization. The sponsors were not directly involved in the study, meaning that they had no role in the study design, data collection, and report writing.
Acknowledgments
We thank the entire EPICO-AEP and EPICO-Colombia working groups for all the entire work. We also thank pediatric residents from the Pediatric Department at Universidad de los Andes that collaborate throughout the process.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2025.1607246/full#supplementary-material
References
1.
World Health Organization. WHO Director-General’s Remarks at the Media Briefing on 2019-nCoV on 11 February 2020 (2020). Available online at: https://www.who.int/director-general/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 (July 12, 2023).
2.
Cobos-Carrascosa E Ballesteros Á Aguilera-Alonso D Mesa JM García-Sánchez P Navarro I et al Manifestations and Clinical Phenotypes Are Not Specific Enough to Predict SARS-CoV-2 Infection in Symptomatic Children. Acta Paediatr Int J Paediatrics (2022) 1–4. 10.1001/jamanetworkopen.2021.43151
3.
Nikolopoulou GB Maltezou HC . COVID-19 in Children: Where Do We Stand?Arch Med Res (2022) 53(1):1–8. 10.1016/j.arcmed.2021.07.002
4.
Parri N Lenge M Cantoni B Arrighini A Romanengo M Urbino A et al COVID-19 in 17 Italian Pediatric Emergency Departments. Pediatrics (2020) 146(6):e20201235. 10.1542/peds.2020-1235
5.
Irwin M Lazarevic B Soled D Adesman A . The COVID-19 Pandemic and its Potential Enduring Impact on Children. Curr Opin Pediatr (2022) 34(1):107–15. 10.1097/MOP.0000000000001097
6.
Ortiz-Prado E Izquierdo-Condoy JS Fernandez-Naranjo R Vasconez J Dávila Rosero MG Revelo-Bastidas D et al The Deadly Impact of COVID-19 Among Children From Latin America: The Case of Ecuador. Front Pediatr (2023) 11:1060311. 10.3389/fped.2023.1060311
7.
Riphagen S Gomez X Gonzalez-Martinez C Wilkinson N Theocharis P . Hyperinflammatory Shock in Children During COVID-19 Pandemic. The Lancet (2020) 395(10237):1607–8. 10.1016/S0140-6736(20)31094-1
8.
Swann OV Holden KA Turtle L Pollock L Fairfield CJ Drake TM et al Clinical Characteristics of Children and Young People Admitted to Hospital With Covid-19 in United Kingdom: Prospective Multicentre Observational Cohort Study. The BMJ (2020) 370:m3249. 10.1136/bmj.m3249
9.
Abraham DR Butters C Abdulbari Yunis N Lishman J Scott C Van Der Zalm MM et al The Impact of SARS-CoV-2 Variants on the Clinical Phenotype and Severity of Multisystem Inflammatory Syndrome in Children in South Africa. Pediatr Infect Dis J (2022) 41(12):E510–2. 10.1097/INF.0000000000003691
10.
Sibbertsen F Glau L Paul K Mir TS Gersting SW Tolosa E et al Phenotypic Analysis of the Pediatric Immune Response to SARS-CoV-2 by Flow Cytometry. Cytometry A (2022) 101(3):220–7. 10.1002/cyto.a.24528
11.
Abbas Q Khalid F Shahbaz FF Khan J Mohsin S Gowa MA et al Clinical and Epidemiological Features of Pediatric Population Hospitalized With COVID-19: A Multicenter Longitudinal Study (March 2020-December 2021) From Pakistan. Lancet Reg Health Southeast Asia (2023) 11:100176. 10.1016/j.lansea.2023.100176
12.
Martin B DeWitt PE Russell S Anand A Bradwell KR Bremer C et al Characteristics, Outcomes, and Severity Risk Factors Associated with SARS-CoV-2 Infection Among Children in the US National COVID Cohort Collaborative. JAMA Netw Open (2022) 5(2):e2143151. 10.1001/jamanetworkopen.2021.43151
13.
Costagliola G Vallario MP Santangelo A Foiadelli T Ragone MC Battini R et al Neurovisual Manifestations in Children With Mild COVID-19: An Association to Remember. Neuroophthalmology (2023) 47(2):75–8. 10.1080/01658107.2023.2174560
14.
Merticariu CI Merticariu M Cobzariu C Mihai MM Dragomir MS . Pediatric Inflammatory Multisystem Syndrome Induced Panuveitis Associated with SARS-CoV- 2 Infection: What the Ophthalmologists Need to Know. Rom J Ophthalmol (2022) 66(2):198–208. 10.22336/rjo.2022.39
15.
Case SM Son MB . COVID-19 in Pediatrics. Rheum Dis Clin North Am (2021) 47(4):797–811. 10.1016/j.rdc.2021.07.006
16.
Atamari-Anahui N Cruz-Nina ND Condori-Huaraka M Nuñez-Paucar H Rondón-Abuhadba EA Ordoñez-Linares ME et al Characterization of Coronavirus Disease 2019 (COVID-19) in Children and Adolescents in Latin American and the Caribbean Countries: A Descriptive Study. Medwave (2020) 20(8):e8025. 10.5867/medwave.2020.08.8025
17.
Burgos C Mendez LM Rodriguez MM Martinez A Sanchez P Tovar C et al What to Look for in Chest X-Rays of Pediatric Patients With COVID-19: Insights from a Colombian Cohort. Pediatr Pulmonology (2025) 60:e27495. 10.1002/ppul.27495
18.
Halabi KC Wang H Leber AL Sánchez PJ Ramilo O Mejias A . Respiratory Syncytial Virus and SARS-CoV-2 Coinfections in Children. Pediatr Pulmonology (2022) 57:3158–60. 10.1002/ppul.26127
19.
Li Y Pillai P Miyake F Nair H . The Role of Viral Co-infections in the Severity of Acute Respiratory Infections Among Children Infected With Respiratory Syncytial Virus (RSV): A Systematic Review and Meta-Analysis. J Glob Health (2020) 10(1):010426. 10.7189/jogh.10.010426
20.
Petat H Gajdos V Angoulvant F Vidalain PO Corbet S Marguet C et al High Frequency of Viral Co-Detections in Acute Bronchiolitis. Viruses (2021) 13(6):990. 10.3390/v13060990
21.
Davies P Evans C Kanthimathinathan HK Lillie J Brierley J Waters G et al Intensive Care Admissions of Children With Paediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2 (PIMS-TS) in the UK: A Multicentre Observational Study. Lancet Child Adolesc Health (2020) 4(9):669–77. 10.1016/S2352-4642(20)30215-7
22.
Tsankov BK Allaire JM Irvine MA Lopez AA Sauvé LJ Vallance BA et al Severe COVID-19 Infection and Pediatric Comorbidities: A Systematic Review and Meta-Analysis. Int J Infect Dis (2021) 103:246–56. 10.1016/j.ijid.2020.11.163
23.
Russell CD Lone NI Baillie JK . Comorbidities, Multimorbidity and COVID-19. Nat Med (2023) 29:334–43. 10.1038/s41591-022-02156-9
24.
Schatz M Hsu JW Zeiger RS Chen W Dorenbaum A Chipps BE et al Phenotypes Determined by Cluster Analysis in Severe or Difficult-To-Treat Asthma. J Allergy Clin Immunol (2014) 133(6):1549–56. 10.1016/j.jaci.2013.10.006
25.
Liao M Li Y Kianifard F Obi E Arcona S . Cluster Analysis and its Application to Healthcare Claims Data: A Study of End-Stage Renal Disease Patients Who Initiated Hemodialysis. BMC Nephrol (2016) 17:25. 10.1186/s12882-016-0238-2
26.
Murtagh F Legendre P . Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion?J Classif (2014) 31(3):274–95. 10.1007/s00357-014-9161-z
27.
Roussos L . The Use of Multiple Correspondence Analysis in Psychometric Item Analysis. Appl Psychol Meas (1987) 11(4):367–77. 10.1109/JBHI.2021.3073605
28.
Machado MB Fajardo TCG De Oliveira LB Quadros Junior ACD Catalan DT Piovesan KC et al Mild and Asymptomatic Coronavirus Disease in Children, Adolescents, and Household Contacts and Prolonged Viral Excretion. Int J Microbiol (2022) 2022:5625104. 10.1155/2022/5625104
29.
Cui X Zhao Z Zhang T Guo W Guo W Zheng J et al A Systematic Review and Meta-Analysis of Children With Coronavirus Disease 2019 (COVID-19). J Med Virol (2021) 93(2):1057–69. 10.1002/jmv.26398
30.
Sobolewska-Pilarczyk M Pokorska-Śpiewak M Stachowiak A Marczyńska M Talarek E Ołdakowska A et al COVID-19 Infections in Infants. Sci Rep (2022) 12(1):1–9. 10.1038/s41598-022-11068-0
31.
Graff K Smith C Silveira L Jung S Curran-Hays S Jarjour J et al Risk Factors for Severe COVID-19 in Children. Pediatr Infect Dis J (2021) 40(4):E137–45. 10.1097/INF.0000000000003043
32.
Kalyanaraman M Anderson MR . COVID-19 in Children. Pediatr Clin N Am (2022) 69(3):547–71. 10.1016/j.pcl.2022.01.013
33.
Diwakar K Sarangi T Srivastava P Tanti SK Swaroop S . Human Adenovirus Infection Causing Hyperinflammatory Syndrome Mimicking Multisystem Inflammatory Syndrome in Children (MIS-C): A Case Report. Cureus (2023) 15(6):6–10. 10.7759/cureus.40239
34.
Gutierrez-Tobar IF Beltran-Arroyave C Díaz A Londoño JP Jimenez KL Zamora CG et al Adenovirus Respiratory Infections Post Pandemic in Colombia: An Old Enemy With Increased Severity in Pediatric Population? Pediatr Infect Dis J (2023) 42(4):e133–e134. 10.1097/INF.0000000000003827
35.
Poeta M Nunziata F Bene MD Morlino F Salatto A Scarano SM et al Diarrhea Is a Hallmark of Inflammation in Pediatric COVID-19. Viruses (2022) 14(12):2723. 10.3390/v14122723
36.
Weng CH Butt WWW Brooks MB Clarke C Jenkins HE Holland SD et al Diagnostic Value of Symptoms for Pediatric SARS-CoV-2 Infection in a Primary Care Setting. PLoS One (2021) 16:e0249980–14. 10.1371/journal.pone.0249980
37.
Westbrook AL Benedit LC Frediani JK Griffiths MA Khan NY Levy JM et al Predictive Value of Isolated Symptoms for Diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Children Tested during Peak Circulation of the Delta Variant. Clin Infect Dis (2022) 75(7):1131–9. 10.1093/cid/ciac112
38.
Hoang A Chorath K Moreira A Evans M Burmeister-Morton F Burmeister F et al COVID-19 in 7780 Pediatric Patients: A Systematic Review. EClinicalMedicine (2020) 24:100433. 10.1016/j.eclinm.2020.100433
Summary
Keywords
COVID-19, pediatrics, inpatients, cluster analysis, SARS-CoV-2 variants
Citation
Sossa-Alarcón MC, Gutiérrez MP, Becerra N, Ortegon LY, David MC, Vanegas MN, Friedrich G, Vásquez-Hoyos P, Mesa-Rubio ML, Navarro-Ramirez LM, Moreno-Lopez S, Baquero OL, Mejía LM, Piñeros JG, Restrepo-Gualteros S, Álvarez-Moreno C, Díaz-Díaz A, Gutierrez-Tobar I, Mesa AC, Bachiller Tuta WR, Galvis Diaz CE, Africano M, Nieto JM, Pérez Camacho PM, Beltrán-Arroyave C, Vivas Trochez R, Gastesi I, Moraleda C, Tagarro García A, Herrero B, Calleja L, Grasa C, Rodriguez P, Melendo S, Soriano-Arandes A, Gómez Pastrana I, García García S, Fumado V and Ramírez Varela A (2025) Phenotypic Variation in Disease Severity Among Hospitalized Pediatric Patients With COVID-19: Assessing the Impact of COVID-19 in the EPICO Study. Int. J. Public Health 70:1607246. doi: 10.3389/ijph.2025.1607246
Received
05 March 2024
Accepted
07 March 2025
Published
18 March 2025
Volume
70 - 2025
Edited by
Taulant Muka, Epistudia, Switzerland
Reviewed by
Two reviewers who chose to remain anonymous
Updates
Copyright
© 2025 Sossa-Alarcón, Gutiérrez, Becerra, Ortegon, David, Vanegas, Friedrich, Vásquez-Hoyos, Mesa-Rubio, Navarro-Ramirez, Moreno-Lopez, Baquero, Mejía, Piñeros, Restrepo-Gualteros, Álvarez-Moreno, Díaz-Díaz, Gutierrez-Tobar, Mesa, Bachiller Tuta, Galvis Diaz, Africano, Nieto, Pérez Camacho, Beltrán-Arroyave, Vivas Trochez, Gastesi, Moraleda, Tagarro García, Herrero, Calleja, Grasa, Rodriguez, Melendo, Soriano-Arandes, Gómez Pastrana, García García, Fumado and Ramírez Varela.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: María Camila Sossa-Alarcón, m.sossaa@uniandes.edu.co
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.