- 1Digestive Oncology Research Center, Digestive Disease Research Institute, Tehran University of Medical Sciences, Tehran, Iran
- 2Liver and Pancreaticobilliary Research Center, Digestive Disease Research Institute, Tehran University of Medical Sciences, Tehran, Iran
- 3Non-Communicable Diseases Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
- 4Medical Doctorate-Master of Public Health (MD-MPH) Program, School of Medicine, Research Center for Traditional Medicine and History of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- 5Cancer Center, Stony Brook Medicine, Stony Brook, NY, United States
- 6Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy
- 7Metabolic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health (NIH), Bethesda, MD, United States
Objectives: To determine the associations between waterpipe use, duration, and intensity of use with prevalence and incidence of metabolic syndrome and its components (increased waist circumference, triglycerides, fasting glucose, blood pressure and decreased high-density lipoprotein cholesterol).
Methods: We conducted cross-sectional and prospective analyses using data from the Pars Cohort Study in southern Iran, encompassing 9,264 participants at the baseline, and 5,002 randomly selected in a repeated follow-up. We used multivariate logistic regression models adjusted for age, sex, education, wealth score, physical activity and cigarette pack-years to report odds ratios (OR) and 95% confidence intervals (CI).
Results: Among 9,264 participants, 3,119 (33.7%) had metabolic syndrome, and 3,482 (37.6%) had ever smoked waterpipe, with both more common in women than in men. In adjusted models, former waterpipe use was significantly associated with prevalence (OR = 1.43, 95% CI: 1.23–1.68) and incidence (OR = 1.57, 95% CI: 1.19–2.06) of the metabolic syndrome while current waterpipe use was not. Past use was associated with increased risk in all components of metabolic syndrome; current use was associated with increases in all except high blood glucose and hypertension. Past waterpipe users had higher waterpipe use intensity (before quitting) in comparison with current users (2.3 vs. 2.0 waterpipes per day, p < 0.01) and had started waterpipe smoking at a younger age (27.2 vs. 30.1 years, p < 0.01).
Conclusion: Waterpipe use was associated with metabolic syndrome and its components, especially among former users potentially due to higher intensity and earlier initiation of use.
Introduction
Waterpipe (also called hookah) is a way of smoking tobacco, which originated in the Middle East [1] but has become increasingly common worldwide, especially among the youth in Europe and the United States [1–3]. Waterpipe use has become a global epidemic with alarming trends, surpassing the prevalence of cigarette smoking in some countries [4]. Waterpipe use has been associated with increased overall and cancer-related mortality [5] and cardiovascular, pulmonary, and endocrine diseases, among others [2, 6]. It has also been associated with obesity [7, 8], hypertension [9, 10], increased fasting blood glucose [10–12], and dyslipidemia [10–12]. However, studies assessing the association of waterpipe use with the aggregation of these abnormalities (i.e., metabolic syndrome) are scarce.
The metabolic syndrome is a growing global health problem which can increase rates of all-cause mortality by up to 1.5 times [13], cardiovascular diseases by twofold [14–16], and type 2 diabetes mellitus by fivefold [14, 16]. The global prevalence of metabolic syndrome is reported at 12.5%–31.4% [17]. Cigarette smoking has been shown to be associated with metabolic syndrome and its components [11, 18–26]. The smoke inhaled during an average session of waterpipe use is equivalent to 39–172 cigarettes [2] and contains similar toxicants [2, 27, 28]. Consecutively, adverse health effects of waterpipe use may be similar to those of cigarette smoking [2, 6, 10, 29]. Even though waterpipe use has been associated with metabolic syndrome in a limited number of studies [9, 11, 30], studies with comprehensive lifetime assessment of exposure and those with a prospective design investigating the risk of metabolic syndrome incidence are lacking.
Pars Cohort Study (PCS) is a study conducted in Fars, a province in the south of Iran, which started in 2012 with a focus on non-communicable diseases [31]. There is a high prevalence of waterpipe use (37.5%) among PCS participants which is higher than the national average [32]. Detailed self-reported tobacco use histories were gathered using a questionnaire previously validated against cotinine levels [27]. These qualities along with the longitudinal nature of the study made PCS a unique platform to explore the association between waterpipe use and metabolic syndrome and its components. This is also the first study on these associations that uses the harmonized global definition of metabolic syndrome proposed in the 2009 Joint Interim Statement [14].
Methods
Pars Cohort Study (PCS) started in 2012 in Valashahr District, a region in the South of Iran, with a population of over 40,000 inhabitants. They are mainly (95%) from Fars or Azari (Qashqai) ethnicities, which are the two major ethnic groups in the country, and live in the city of Valashahr and 93 villages. The sample of this study consisted of all eligible adults between 40 and 75. The response rate was 95% among residents who were invited from the city and nearby villages [31]. In a nested study, 5,002 participants were randomly selected for three repeated follow-up sessions with detailed laboratory data and drug histories in 2016, 2018, and 2021 [33]. Pars Cohort Study and its protocols were approved by ethical committees of Digestive Diseases Research Institute of Tehran University of Medical Sciences and Shiraz University of Medical Sciences.
At baseline, a general questionnaire was administered by trained interviewers which included self-reported personal, demographic and lifestyle characteristics such as education, questions addressing wealth level, physical activity, tobacco and alcohol use, plus medical history including disease and medication history. A composite wealth score was calculated based on house ownership, house size, and appliance ownership using multiple correspondence analysis as described before [34]. The wealth score was categorized into quartiles with 1 being the poorest to 4 being the wealthiest participants. Education was classified based on the years of formal schooling into four groups: “none,” “1–5 years,” “6–8 years” and “over 8 years.” Physical activity was determined using the Metabolic Equivalent of Task (MET, in minutes per week) and classified into quartiles.
We also collected a complete self-reported tobacco use profile at baseline, including the lifetime start and stop ages, daily consumption amounts, and frequency of use at each age using a detailed questionnaire (Supplementary Table S1). The questionnaire was validated in a previous study, showing 95.8% accuracy compared with urinary cotinine as the gold standard [27]. For cigarette and waterpipe use (each separately) ever users (vs. never users) were those who smoked cigarette/waterpipe at least once a week for 6 months and former users (vs. current users) were those who had stopped smoking cigarette/waterpipe at least 1 year before enrolling in this study.
Average waterpipe intensity (times per day) was calculated based on the individual’s average lifetime use and categorized into three groups: less than once a day, once or twice a day, and more than twice a day. Lifetime duration for waterpipe use was calculated as the number of years between the first use and their quitting time, or their current age (if they were still using), then categorized into tertiles: 10 years or fewer, 11–25 years, and more than 25 years.
We calculated cumulative cigarette use as pack-years [35, 36]by multiplying the duration of use by the average number of packs per day, across all periods of use. For example, someone who smoked 2 cigarette packs per day for 10 years, and someone who smoked 1 pack for 20 years both smoked a cumulative amount of 20 pack-years. We also calculated waterpipe cumulative use as waterpipe-years in the same way, and used the number of times per day instead of packs in the formula, as described before [5, 37]. Then we categorized the cumulative use into tertiles: 10 or fewer, 11–28, and more than 28 for cigarette pack-years; 10 or fewer, 11–48 and more than 48 for waterpipe-years.
Anthropologic indices (weight, height, waist and hip circumference) were measured and recorded by trained nurses and blood pressure was measured by a physician using a standard protocol [38]. BMI was calculated as weight (in kilograms) divided by squared height (in meters) and then classified based on WHO guidelines. Waist circumference was measured at umbilicus level, then divided into sex-specific quartiles. For each participant fasting blood glucose (FBG) and lipid profile were measured using commercial kits. The anthropologic measurements, blood pressure and laboratory measurements were repeated at the follow-up sessions along with updated drug histories.
We defined metabolic syndrome based on the 2009 Joint Interim Statement from AHA/NHLBI, World Heart Federation, International Atherosclerotic Society, and International Association for the Study of Obesity [14] which introduces a globally harmonized definition for metabolic syndrome. In this definition, metabolic syndrome is defined as the presence of 3 or more of the following criteria: 1. Elevated waist circumference (≥80 cm in women and ≥94 cm in men), 2. Elevated blood pressure (systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥85 mmHg or antihypertensive drug treatment), 3. Elevated fasting glucose (fasting blood glucose ≥100 mg/dL or drug treatment of elevated glucose), 4. Reduced high-density lipoprotein cholesterol (HDL-C) (<50 mg/dL in women and <40 mg/dL in men or drug treatment for reduced HDL-C), 5. Elevated triglycerides (≥150 mg/dL or drug treatment for elevated triglycerides).
Statistical Analysis
Descriptive data are presented as frequencies and percentages for categorical variables, and means and standard deviations for continuous variables. The chi-square test was used to compare categorical variables, and independent sample t-test was used for continuous variables. Our analysis consisted of a cross-sectional analysis using prevalent metabolic syndrome (for which we used the baseline data) and a prospective analysis to assess the risk of incident metabolic syndrome. Incident cases were defined as those who had a new diagnosis of metabolic syndrome during any of the three follow-up sessions and did not have metabolic syndrome at baseline. We used multiple logistic regression models to determine the adjusted odds ratios (ORs) and 95% confidence intervals (CIs). The main independent variables were waterpipe use (never, past, current), waterpipe use intensity (times per day), waterpipe use lifetime duration (in years), and cumulative waterpipe use (waterpipe-years) which were each used in separate models. Since past and current waterpipe users had different intensity and duration of use, two strategies were adopted: 1. Each correlate (intensity, duration and cumulative use) was stratified by past and current status, 2. The models including current and past waterpipe use were additionally adjusted for use intensity. Exclusive waterpipe use was also assessed in a separate model where cigarette smokers were excluded. The reference category in all these models (five models total for prevalence and five models total for incidence) was never waterpipe users. We adjusted the models for age, sex, education, wealth score, physical activity and cigarette pack-years. To make the models more parsimonious, we did not adjust our models for ethnicity and alcohol use as they did not have a significant effect on the estimates. A directed acyclic graph (DAG) was used to illustrate the relationships between variables and determine the confounding variables (Supplementary Figure S1). As both metabolic syndrome and waterpipe use varied significantly between men and women all analyses were also repeated stratified by sex.
The same adjusted models were used for the association of ever waterpipe use and each metabolic syndrome component (high blood pressure, high blood glucose, low HDL, high triglycerides, and high waist circumference as defined before). For prospective analysis, incident cases of each component were defined as new occurrences of the component during the follow-up together with the absence of the component at baseline.
We conducted two separate sensitivity analyses to investigate the possibility of reverse causation in which we excluded subjects with a self-reported history of chronic diseases (cardiovascular diseases, cerebrovascular accident, chronic obstructive pulmonary disease, chronic renal failure, jaundice and liver disease) once with and once without a history of cigarette smoking.
We used Stata software version 17 (StataCorp Inc., College Station, TX, United States) for statistical analyses. A two-sided p-value of <0.05 was considered statistically significant.
Results
As seen in Table 1, among 9,264 participants, 4,276 (46.2%) were men and 4,988 (53.8%) were women, with a mean age of 52.6 (SD = 9.7). A total number of 3,119 (33.6%) fulfilled the criteria for the metabolic syndrome (MetS), including 965 men (22.6% of all men) and 2,154 women (43.2% of all women). The prevalence of the metabolic syndrome components from the most to least common at the baseline were as follows: high waist circumference (61.7%), high blood sugar (42.9%), high serum triglyceride (39.6%), high blood pressure (32.1%), and low serum HDL (19.7%). All components were significantly more common in women than in men (p < 0.01) (Supplementary Table S2).
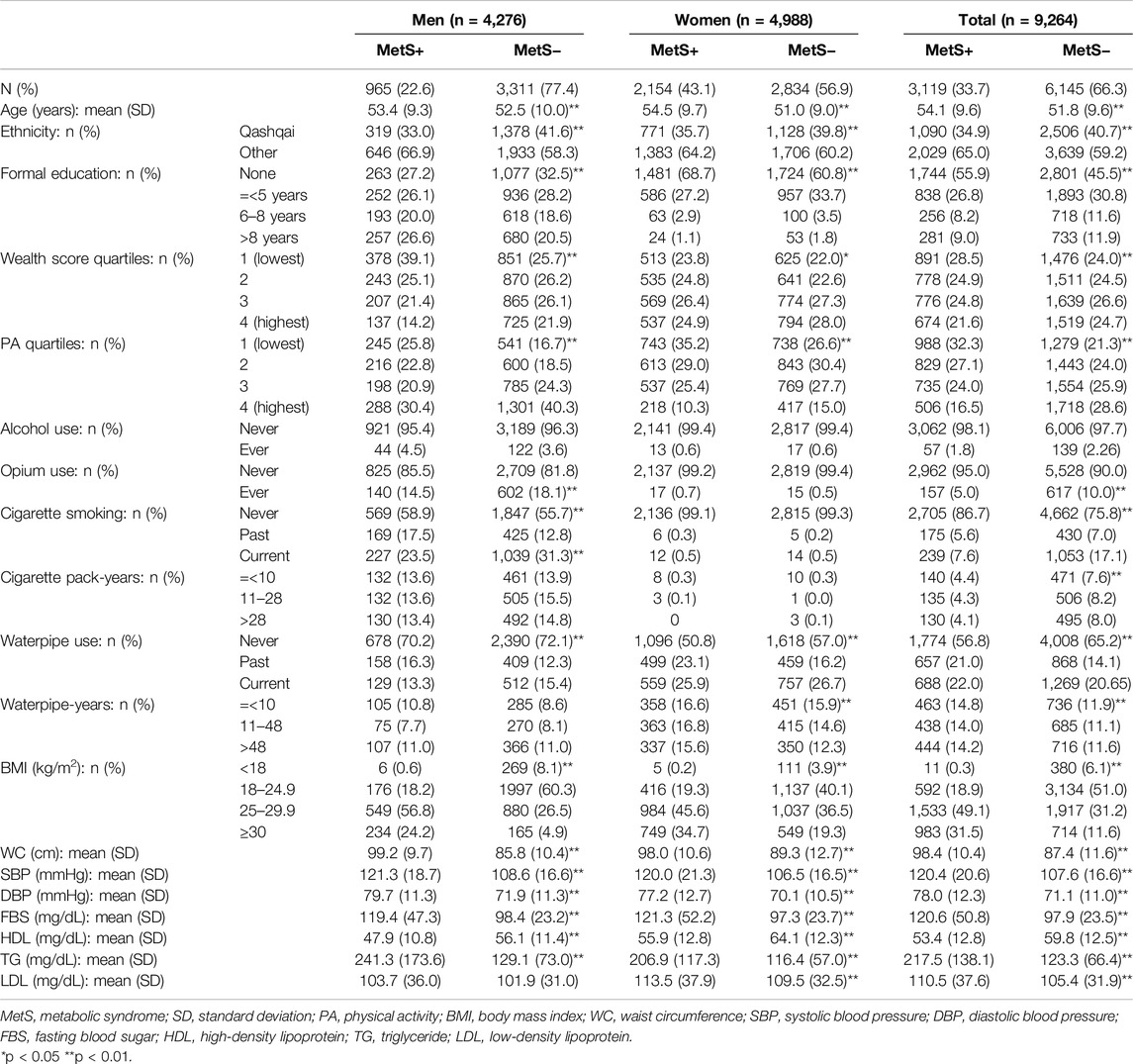
Table 1. Baseline characteristics of the Pars cohort study population by metabolic syndrome and sex (Prevalence and Incidence of Metabolic Syndrome and Its Components among Waterpipe Users, Iran, 2024).
The metabolic syndrome had a positive significant association with age, it was more common among ethnicities other than Qashqai (the most common ethnic group in this population), and it showed an inverse significant relationship with education, wealth score and physical activity in both men and women. Prevalent metabolic syndrome was inversely associated with ever opium use and cigarette smoking. We did not find any associations between the metabolic syndrome and alcohol use (Table 1).
Almost all the tobacco used in this population was either in the form of cigarettes or waterpipe, except for 13 people who also chewed tobacco. A total number of 3,482 (37.6%) people reported ever waterpipe use (past and current), including 1,208 men (28.3% of all men) and 2,274 women (45.6% of all women). Waterpipe use was significantly more common in women (p < 0.01) (Supplementary Table S2). Cigarette smoking was rare among women (past: 0.2%, current: 0.5%) compared to men (past: 13.8%, current: 29.6%). Waterpipe users were significantly older, wealthier and less educated than non-users. Waterpipe use was more common among past cigarette smokers compared with never and current cigarette smokers and was inversely associated with cigarette pack-years (Supplementary Table S3). Past waterpipe users had higher waterpipe use intensity (before quitting) in comparison with current users (2.3 vs. 2.0 waterpipes per day, p < 0.01) and had started waterpipe smoking at a younger age (27.2 vs. 30.1 years, p < 0.01).
The findings of the logistic regression analysis for prevalent and incident metabolic syndrome are presented in Table 2, consisting of the total population and sex-stratified estimates. Below, the total population estimates are explained, with sex-stratified estimates detailed if discordant.
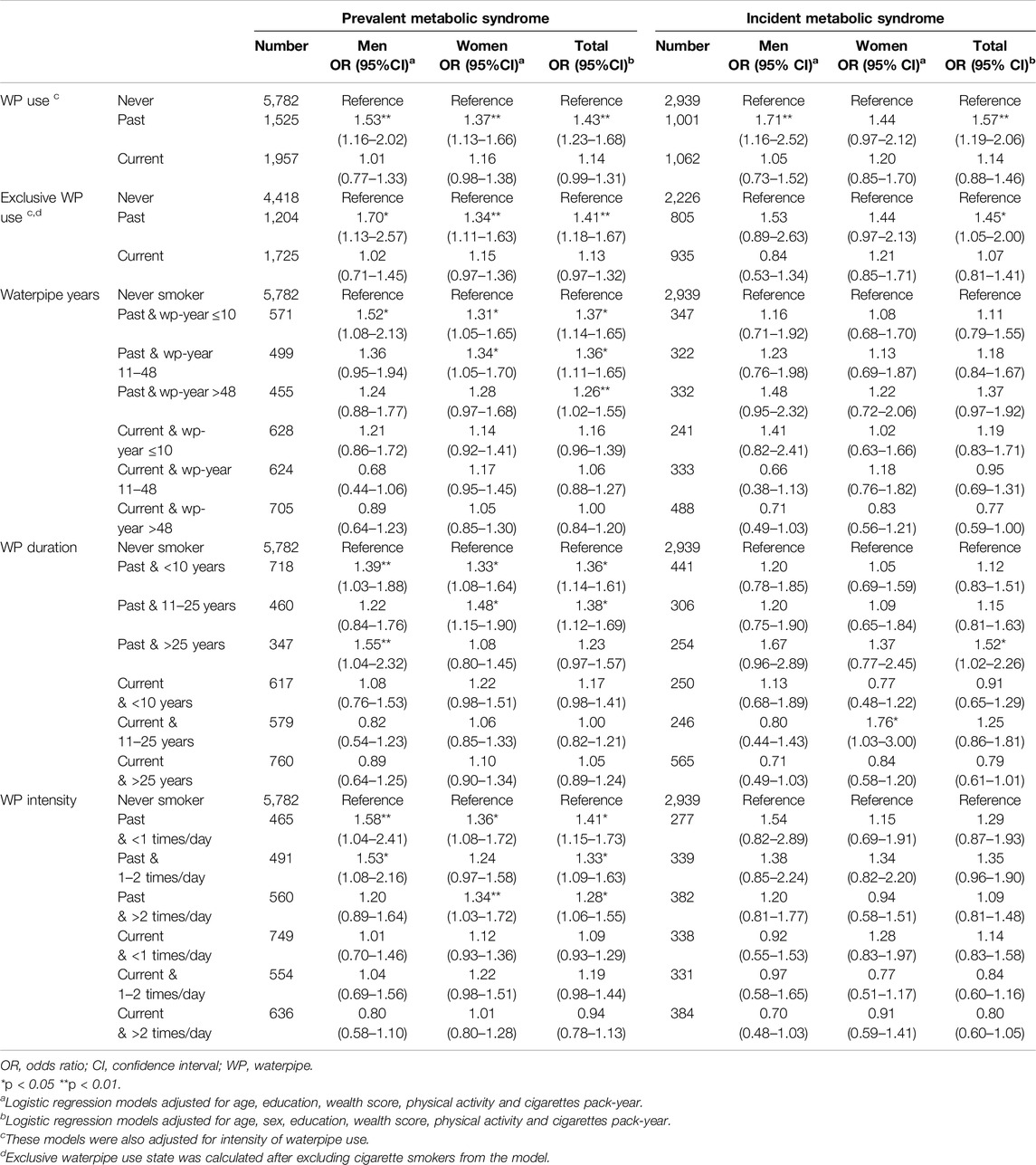
Table 2. The association of correlates of waterpipe use with prevalence and incidence of metabolic syndrome in adjusted logistic regression models (Prevalence and Incidence of Metabolic Syndrome and Its Components among Waterpipe Users, Iran, 2024).
Past and Current Waterpipe Use
There were significant positive associations between past waterpipe use and prevalent metabolic syndrome (OR = 1.43, 95% CI: 1.23–1.68) and incident metabolic syndrome (OR = 1.57, 95% CI: 1.19–2.06), although the association with incidence was not significant among women. After the exclusion of cigarette smokers, the association of past use with metabolic syndrome remained significant for prevalence (OR = 1.41, 95% CI: 1.18–1.67), but the association with incidence was only significant in the total population (OR = 1.45, 95% CI: 1.05–2.00) and not the sex strata. When we excluded individuals with a history of chronic diseases as a sensitivity analysis (Supplementary Table S4), the association between past waterpipe smoking and prevalent metabolic syndrome weakened (OR = 1.33, 95% CI: 1.11–1.60).
There were no significant associations between current waterpipe use and the metabolic syndrome. In the sensitivity analysis excluding those with a history of chronic diseases (Supplementary Table S4), current use showed a statistically significant association (OR = 1.21; 95% CI: 1.04–1.42) albeit non-significant among men.
Cumulative Waterpipe Use
There were significant associations between all levels of cumulative waterpipe use and prevalence of metabolic syndrome among past waterpipe users. In women, only the association for former users with above 48 waterpipe-years was not statistically significant, while in men only the association between waterpipe-years ≤10 and the prevalent metabolic syndrome reached statistical significance. Associations between waterpipe-years and incident metabolic syndrome were similar but insignificant. No significant association was found between waterpipe cumulative use and metabolic syndrome among current users.
Duration of Waterpipe Use
Duration of use was significantly associated with prevalent metabolic syndrome among past users but not current users. Less than 10 years and 11–25 years of use among past waterpipe users were associated with prevalent metabolic syndrome with odds ratios of 1.36 (95% CI: 1.14–0.61) and 1.38 (95% CI: 1.12–1.69), respectively with the latter not statistically significant among men. Over 25 years of duration of use among former waterpipe users was significantly associated with metabolic syndrome incidence only in the total population (OR = 1.52, 95% CI: 1.02–2.26) but not among men and women separately.
Intensity of Waterpipe Use
All categories of intensity were associated with prevalent metabolic syndrome among past users but not current users. Daily intensities of less than 1 session, 1–2 times, and above 2 times were associated with prevalent metabolic syndrome with odds ratios of 1.41 (95% CI: 1.15–1.73), 1.33 (95% CI: 1.09–1.63), and 1.28 (95% CI: 1.06–1.55). Sex-stratified results of former users were similar to that of the total population except for an intensity of >2 times per day among men and 1–2 times per day among women. While no significant association was found between intensity of use and metabolic syndrome incidence, the associations were generally in accordance with those of the prevalence assessment.
As Table 3 shows, in adjusted models, past waterpipe use was significantly associated with increased prevalence of high waist circumferences (OR = 1.81, 95% CI: 1.49–2.19), prevalence and incidence of low HDL (OR = 1.25, 95% CI: 1.05–1.49 and OR = 1.34, 95% CI: 1.07–1.69, respectively), the prevalence and incidence of high triglycerides (OR = 1.17, 95% CI: 1.01–1.36 and OR = 1.39, 95% CI: 1.08–1.78, respectively), prevalence and incidence of increased blood glucose (OR = 1.17, 95% CI: 1.01–1.36 and OR = 1.69, 95% CI: 1.29–2.21), and prevalence of high blood pressure (OR = 1.44, 95% CI: 1.23–1.69). Current waterpipe use was associated with increased prevalence of high waist circumferences (OR = 1.42, 95% CI: 1.20–1.69), prevalence and incidence of low HDL (OR = 1.20, 95% CI: 1.02–1.40 and OR = 1.32, 95% CI: 1.06–1.64, respectively), and incidence of high serum triglycerides (OR = 1.39, 95% CI: 1.09–1.77). Current use was also associated with a reduction in the prevalence of high blood pressure (OR = 0.85, 95% CI: 0.73–0.98).
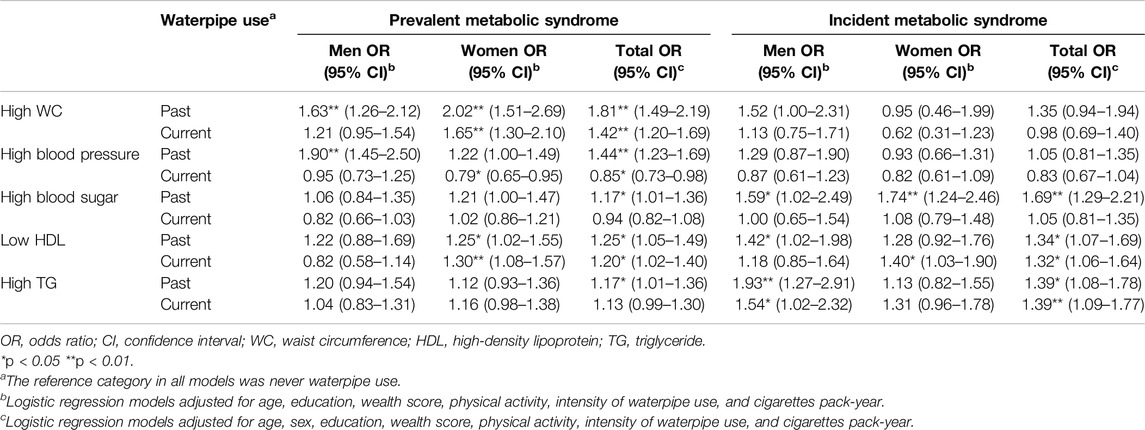
Table 3. The association of past and current waterpipe use with components of metabolic syndrome in adjusted logistic regression models for prevalent and incident cases of metabolic syndrome by sex (Prevalence and Incidence of Metabolic Syndrome and Its Components among Waterpipe Users, Iran, 2024).
Discussion
In this population of Southern Iran, both the metabolic syndrome and waterpipe smoking were more common among women. Past waterpipe smokers had higher intensity of use and had started smoking at lower ages compared with current waterpipe smokers. We found a statistically significant association between waterpipe use, its intensity and duration and both prevalent and incident metabolic syndrome among former users. When we excluded individuals with a history of chronic diseases, these associations weakened. Former waterpipe use compared to never use is associated with an increased likelihood of all metabolic syndrome components and with the risk of incidence for increased blood glucose, increased triglyceride, and low HDL. Current waterpipe use was associated with an increase in the incidence of high triglyceride and low HDL, an increase in the prevalence of high waist circumference and low HDL, along with a decrease in prevalence of hypertension.
Women were more likely to use waterpipe in our population, while in many countries around the world the rate of use is higher among men [39]. Studies have shown that women in West Asia and North Africa prefer using waterpipe to cigarettes due to social acceptance [40, 41]. About one-third of our participants had the metabolic syndrome (33.6%). It is not easy to determine the global prevalence of the metabolic syndrome due to different definitions used for it [42], but in the year 2022, it was estimated to be 31.4% among the general adult population based on the Joint Interim Statement used in our study [17]. According to our results, metabolic syndrome was more common among women, which was consistent with previous studies from Iran [43, 44]. In our population, women were physically less active and had higher rates of central obesity compared to men (85.5% in women vs. 34.1% in men).
Previous studies on cigarette smoking have shown a positive association with the metabolic syndrome [20, 21, 23–26] and this association was dose-dependent in some studies [18, 22]. This association was also seen in both direct tobacco smoking and exposure to second-hand tobacco smoke [45]. According to our results, past waterpipe use was associated with the metabolic syndrome, but current waterpipe use was not. This was consistent with a previous study conducted by Soltani et al, who investigated the relationship between waterpipe smoking and the metabolic syndrome in individuals 60 or older, and found that former waterpipe users had higher rates of the metabolic syndrome in comparison with never and current users [30]. Another study by Soflaei et al showed that waterpipe use was associated with increased prevalence of metabolic syndrome while cigarette smoking was not [11]. Other studies have also showed significant associations between current waterpipe use and the metabolic syndrome [29]. The reason for these differences is not clear, but past waterpipe smokers in our study had a significantly higher intensity of waterpipe use and had started smoking waterpipe at younger ages than current users which may have led the associations to be stronger in past users. In all likelihood, heavier use and earlier start may have led to adverse health reactions and more doctors’ visits recommending quitting, as also shown in a previously conducted study on quitting patterns among cigarette smokers above the age of 44 [46]. This potential reverse causation effect was also observed with the weakening of the associations among past waterpipe users with the exclusion of individuals with a history of chronic diseases.
Cigarette smoking has been shown to affect body fat distribution. Cigarette smokers have higher rates of central obesity despite lower BMI in comparison with non-smokers [47]. Past smokers have higher rates of increased waist circumference and lowered HDL in comparison with never smokers [48]. The effect of quitting smoking on weight gain and has been shown in several studies [26] and can explain the stronger associations between past waterpipe use, compared with current use, and both waist circumference and metabolic syndrome. In our study, both past and current waterpipe users had higher waist circumferences compared with non-users, as well as higher levels of serum triglycerides and lower HDL. Soltani et al found associations between past waterpipe use with central obesity and current waterpipe use with decreased HDL levels [30]. Soflaei et al showed an association between current waterpipe use and both increased waist circumference and decreased HDL levels [11]. The positive relationship between waterpipe use and obesity has also been shown in other studies [9, 49]. Another study showed that daily waterpipe users had a three-fold higher risk for obesity in comparison with never-users [50]. Increased risk of metabolic syndrome among tobacco users may also be due to unhealthy lifestyle and lack of physical activity, but our analyses were adjusted for physical activity.
Previous results on the relationship between waterpipe use and blood pressure have been controversial. Some studies have shown an association between waterpipe use and high blood pressure [9, 51, 52]. On the other hand, there have been reports of reduction in heart rate and blood pressure in waterpipe users [30, 53, 54]. We showed that while past waterpipe users had higher blood pressure than non-users, current waterpipe users had lower blood pressure. A study conducted on awareness and risk factors of hypertension in Iran showed that compared to non-smokers, current but not past smokers had lower blood pressures, and past smokers had higher awareness of their health condition [55], so they might have quit smoking because of the health consequences. Furthermore, another study on preventive lifestyle behaviours in hypertension conducted in Canada proposed a positive association between perceiving hypertension as a health hazard due to smoking cigarettes and quitting smoking to control blood pressure [56].
We studied a population with relatively high rates of both waterpipe smoking and the metabolic syndrome. We used longitudinal data for metabolic syndrome incidence and a validated self-reported comprehensive tobacco use history at baseline for exposure assessment.
Limitations
While we found significant associations between waterpipe use and metabolic syndrome, the associations with past waterpipe use may be partly due to reverse causation or residual confounding. Though national surveys confirm the low proportion of alcohol use in this population [57], we cannot completely rule out the likelihood of underreporting due to religious beliefs. Tobacco use histories were not based on objective methods, however, our self-report questionnaire has been validated previously against cotinine values.
Conclusion
We found significant associations between waterpipe smoking and the metabolic syndrome components, as well as the syndrome itself. Given the alarming increase in the popularity of waterpipe use, our findings build on the diverse adverse health impacts of this method of tobacco use, highlighting the need for preventive health strategies. Further experimental research on the potential mechanism of the effects of waterpipe use on metabolic syndrome and larger prospective studies are warranted to clarify the observed associations.
Ethics Statement
The Pars Cohort Study and its protocols were approved by the ethical committees of Digestive Diseases Research Institute of Tehran University of Medical Sciences and Shiraz University of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author Contributions
MN authored parts of the original draft, contributed to data curation, conducted formal analysis and reviewed and edited the final manuscript. CA conceptualized the study and helped in methodology and validation. NF helped in conceptualizing the study, investigation and validation. HP was one of the project administrators and helped with supervision and data curation. RM the coauthor of the study, was one of the supervisors and project administrators, and also acquired the funding. AE another coauthor of the study, was project administrator and supervisor, and also participated in data analysis, investigation, methodology and writing the original draft. YS was the main author, participated in data analysis, investigation and methodology, did the literature review and wrote the original draft. SA helped with data analysis and editing the draft. HM helped in data curation and providing the resources. FM helped with data curation. AG helped with data curation and providing the resources. PB participated in methodology and validation of the study. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The field work was funded by the Shiraz University of Medical Sciences (910210). This manuscript was supported in part by the Intramural Research Program of the US National Cancer Institute, NIH.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2024.1607156/full#supplementary-material
References
2. Soule, EK, Lipato, T, and Eissenberg, T. Waterpipe Tobacco-Smoking: A New Smoking Epidemic Among the Young? Curr Pulmonol Rep (2015) 4(4):163–72. doi:10.1007/s13665-015-0124-6
3. Jawad, M, Charide, R, Waziry, R, Darzi, A, Ballout, RA, and Akl, EA. The Prevalence and Trends of Waterpipe Tobacco Smoking: A Systematic Review. PLOS ONE (2018) 13(2):e0192191. doi:10.1371/journal.pone.0192191
4. Maziak, W, Taleb, ZB, Bahelah, R, Islam, F, Jaber, R, Auf, R, et al. The Global Epidemiology of Waterpipe Smoking. Tob Control (2015) 24(Suppl. 1):i3–i12. doi:10.1136/tobaccocontrol-2014-051903
5. Etemadi, A, Khademi, H, Kamangar, F, Freedman, ND, Abnet, CC, Brennan, P, et al. Hazards of Cigarettes, Smokeless Tobacco, and Waterpipe in a Middle Eastern Population: A Cohort Study of 50,000 Individuals From Iran. Tob Control (2017) 26(6):674–82. doi:10.1136/tobaccocontrol-2016-053245
6. El-Zaatari, ZM, Chami, HA, and Zaatari, GS. Health Effects Associated With Waterpipe Smoking. Tob Control (2015) 24(Suppl. 1):i31–43. doi:10.1136/tobaccocontrol-2014-051908
7. Alkeilani, AA, Khalil, AA, Azzan, AM, Al-Khal, NA, Al-Nabit, NH, Talab, OM, et al. Association Between Waterpipe Smoking and Obesity: Population-Based Study in Qatar. Tob Induc Dis (2022) 20:06. doi:10.18332/tid/143878
8. Baalbaki, R, Itani, L, El Kebbi, L, Dehni, R, Abbas, N, Farsakouri, R, et al. Association Between Smoking Hookahs (Shishas) and Higher Risk of Obesity: A Systematic Review of Population-Based Studies. J Cardiovasc Dev Dis (2019) 6(2):23. doi:10.3390/jcdd6020023
9. Shafique, K, Mirza, SS, Mughal, MK, Arain, ZI, Khan, NA, Tareen, MF, et al. Water-Pipe Smoking and Metabolic Syndrome: A Population-Based Study. PLoS ONE (2012) 7(7):e39734. doi:10.1371/journal.pone.0039734
10. Mahfooz, K, Vasavada, AM, Joshi, A, Pichuthirumalai, S, Andani, R, Rajotia, A, et al. Waterpipe Use and its Cardiovascular Effects: A Systematic Review and Meta-Analysis of Case-Control, Cross-Sectional, and Non-Randomized Studies. Cureus (2023) 15(2):e34802. doi:10.7759/cureus.34802
11. Saffar, SS, Darroudi, S, Tayefi, M, Nosrati Tirkani, A, Moohebati, M, Ebrahimi, M, et al. Hookah Smoking Is Strongly Associated With Diabetes Mellitus, Metabolic Syndrome and Obesity: A Population-Based Study. Diabetol Metab Syndr (2018) 10(1):33. doi:10.1186/s13098-018-0335-4
12. Al Ali, R, Vukadinović, D, Maziak, W, Katmeh, L, Schwarz, V, Mahfoud, F, et al. Cardiovascular Effects of Waterpipe Smoking: A Systematic Review and Meta-Analysis. Rev Cardiovasc Med (2020) 21(3):453–68. doi:10.31083/j.rcm.2020.03.135
13. Mottillo, S, Filion, KB, Genest, J, Joseph, L, Pilote, L, Poirier, P, et al. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. J Am Coll Cardiol (2010) 56(14):1113–32. doi:10.1016/j.jacc.2010.05.034
14. Alberti, KGMM, Eckel, RH, Grundy, SM, Zimmet, PZ, Cleeman, JI, Donato, KA, et al. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation (2009) 120(16):1640–5. doi:10.1161/CIRCULATIONAHA.109.192644
15. Mohanan, P. Metabolic Syndrome in the Indian Population: Public Health Implications. Hypertens J (2016) 2(1):1–6. doi:10.5005/jp-journals-10043-0021
16. Wilson, PWF, D’Agostino, RB, Parise, H, Sullivan, L, and Meigs, JB. Metabolic Syndrome as a Precursor of Cardiovascular Disease and Type 2 Diabetes Mellitus. Circulation (2005) 112(20):3066–72. doi:10.1161/CIRCULATIONAHA.105.539528
17. Noubiap, JJ, Nansseu, JR, Lontchi-Yimagou, E, Nkeck, JR, Nyaga, UF, Ngouo, AT, et al. Geographic Distribution of Metabolic Syndrome and its Components in the General Adult Population: A Meta-Analysis of Global Data From 28 Million Individuals. Diabetes Res Clin Pract (2022) 188:109924. doi:10.1016/j.diabres.2022.109924
18. Chen, CC, Li, TC, Chang, PC, Liu, CS, Lin, WY, Wu, MT, et al. Association Among Cigarette Smoking, Metabolic Syndrome, and its Individual Components: The Metabolic Syndrome Study in Taiwan. Metabolism (2008) 57(4):544–8. doi:10.1016/j.metabol.2007.11.018
19. Masulli, M, and Vaccaro, O. Association Between Cigarette Smoking and Metabolic Syndrome. Diabetes Care (2006) 29(2):482–3. doi:10.2337/diacare.29.02.06.dc05-2077
20. Miyatake, N, Wada, J, Kawasaki, Y, Nishii, K, Makino, H, and Numata, T. Relationship Between Metabolic Syndrome and Cigarette Smoking in the Japanese Population. Intern Med Tokyo Jpn (2006) 45(18):1039–43. doi:10.2169/internalmedicine.45.1850
21. Nakanishi, N, Takatorige, T, and Suzuki, K. Cigarette Smoking and the Risk of the Metabolic Syndrome in Middle-Aged Japanese Male Office Workers. Ind Health (2005) 43(2):295–301. doi:10.2486/indhealth.43.295
22. Cena, H, Tesone, A, Niniano, R, Cerveri, I, Roggi, C, and Turconi, G. Prevalence Rate of Metabolic Syndrome in a Group of Light and Heavy Smokers. Diabetol Metab Syndr (2013) 5(1):28. doi:10.1186/1758-5996-5-28
23. Weitzman, M, Cook, S, Auinger, P, Florin, TA, Daniels, S, Nguyen, M, et al. Tobacco Smoke Exposure Is Associated With the Metabolic Syndrome in Adolescents. Circulation (2005) 112(6):862–9. doi:10.1161/CIRCULATIONAHA.104.520650
24. Kawada, T, Otsuka, T, Inagaki, H, Wakayama, Y, Li, Q, Li, YJ, et al. Association of Smoking Status, Insulin Resistance, Body Mass Index, and Metabolic Syndrome in Workers: A 1-Year Follow-Up Study. Obes Res Clin Pract (2010) 4(3):e163–246. doi:10.1016/j.orcp.2009.12.004
25. Geslain-Biquez, C, Vol, S, Tichet, J, Caradec, A, D’Hour, A, Balkau, B, et al. The Metabolic Syndrome in Smokers. The D.E.S.I.R. Study. Diabetes Metab (2003) 29(3):226–34. doi:10.1016/s1262-3636(07)70031-9
26. Kim, HJ, and Cho, YJ. Smoking Cessation and Risk of Metabolic Syndrome: A Meta-Analysis. Medicine (Baltimore) (2024) 103(22):e38328. doi:10.1097/md.0000000000038328
27. Etemadi, A, Poustchi, H, Chang, CM, Blount, BC, Calafat, AM, Wang, L, et al. Urinary Biomarkers of Carcinogenic Exposure Among Cigarette, Waterpipe and Smokeless Tobacco Users and Never Users of Tobacco in the Golestan Cohort Study. Cancer Epidemiol Biomarkers Prev (2019) 28(2):337–47. doi:10.1158/1055-9965.EPI-18-0743
28. Cobb, CO, Shihadeh, A, Weaver, MF, and Eissenberg, T. Waterpipe Tobacco Smoking and Cigarette Smoking: A Direct Comparison of Toxicant Exposure and Subjective Effects. Nicotine Tob Res (2011) 13(2):78–87. doi:10.1093/ntr/ntq212
29. Waziry, R, Jawad, M, Ballout, RA, Al Akel, M, and Akl, EA. The Effects of Waterpipe Tobacco Smoking on Health Outcomes: An Updated Systematic Review and Meta-Analysis. Int J Epidemiol (2017) 46(1):32–43. doi:10.1093/ije/dyw021
30. Soltani, D, Heshmat, R, Vasheghani-Farahani, A, Fahimfar, N, Masoudkabir, F, Ashraf, H, et al. The Association Between Waterpipe Smoking and Metabolic Syndrome: A Cross-Sectional Study of the Bushehr Elderly Health Program. Biomed Env Sci (2021) 34(11):910–5. doi:10.3967/bes2021.125
31. Gandomkar, A, Poustchi, H, Moini, M, Moghadami, M, Imanieh, H, Fattahi, MR, et al. Pars Cohort Study of Non-Communicable Diseases in Iran: Protocol and Preliminary Results. Int J Public Health (2017) 62(3):397–406. doi:10.1007/s00038-016-0848-2
32. Abbasi-Kangevari, M, Ghanbari, A, Fattahi, N, Malekpour, MR, Masinaei, M, Ahmadi, N, et al. Tobacco Consumption Patterns Among Iranian Adults: A National and Sub-National Update From the STEPS Survey 2021. Sci Rep (2023) 13(1):10272. doi:10.1038/s41598-023-37299-3
33. Malekzadeh, F, Gandomkar, A, Poustchi, H, Etemadi, A, Roshandel, G, Attar, A, et al. Effectiveness of Polypill for Primary and Secondary Prevention of Cardiovascular Disease: A Pragmatic Cluster-Randomised Controlled Trial (PolyPars). Heart Br Card Soc (2024) 110:940–6. doi:10.1136/heartjnl-2023-323614
34. Islami, F, Kamangar, F, Nasrollahzadeh, D, Aghcheli, K, Sotoudeh, M, Abedi-Ardekani, B, et al. Socio-Economic Status and Oesophageal Cancer: Results From a Population-Based Case-Control Study in a High-Risk Area. Int J Epidemiol (2009) 38(4):978–88. doi:10.1093/ije/dyp195
35. Bernaards, CM, Twisk, JWR, Snel, J, Van Mechelen, W, and Kemper, HCG. Is Calculating Pack-Years Retrospectively a Valid Method to Estimate Life-Time Tobacco Smoking? A Comparison Between Prospectively Calculated Pack-Years and Retrospectively Calculated Pack-Years. Addiction (2001) 96(11):1653–61. doi:10.1046/j.1360-0443.2001.9611165311.x
36. Thomas, DC. Invited Commentary: Is It Time to Retire the “Pack-Years” Variable? Maybe Not. Am J Epidemiol (2014) 179(3):299–302. doi:10.1093/aje/kwt274
37. Maziak, W, Taleb, ZB, Jawad, M, Afifi, R, Nakkash, R, Akl, EA, et al. Consensus Statement on Assessment of Waterpipe Smoking in Epidemiological Studies. Tob Control (2017) 26(3):338–43. doi:10.1136/tobaccocontrol-2016-052958
38. Muntner, P, Shimbo, D, Carey, RM, Charleston, JB, Gaillard, T, Misra, S, et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension (2019) 73(5):e35–66. doi:10.1161/HYP.0000000000000087
39. Bhatnagar, A, Maziak, W, Eissenberg, T, Ward, KD, Thurston, G, King, BA, et al. Water Pipe (Hookah) Smoking and Cardiovascular Disease Risk: A Scientific Statement From the American Heart Association. Circulation (2019) 139(19):e917–36. doi:10.1161/CIR.0000000000000671
40. Baheiraei, A, Shahbazi Sighaldeh, S, Ebadi, A, Kelishadi, R, and Majdzadeh, R. Factors That Contribute in the First Hookah Smoking Trial by Women: A Qualitative Study From Iran. Iran J Public Health (2015) 44(1):100–10.
41. Nakkash, R, Khader, Y, Chalak, A, Abla, R, Abu-Rmeileh, NME, Mostafa, A, et al. Prevalence of Cigarette and Waterpipe Tobacco Smoking Among Adults in Three Eastern Mediterranean Countries: A Cross-Sectional Household Survey. BMJ Open (2022) 12(3):e055201. doi:10.1136/bmjopen-2021-055201
42. O’Neill, S, and O’Driscoll, L. Metabolic Syndrome: A Closer Look at the Growing Epidemic and its Associated Pathologies. Obes Rev (2015) 16(1):1–12. doi:10.1111/obr.12229
43. Azizi, F, Salehi, P, Etemadi, A, and Zahedi-Asl, S. Prevalence of Metabolic Syndrome in an Urban Population: Tehran Lipid and Glucose Study. Diabetes Res Clin Pract (2003) 61(1):29–37. doi:10.1016/s0168-8227(03)00066-4
44. Delavari, A, Forouzanfar, MH, Alikhani, S, Sharifian, A, and Kelishadi, R. First Nationwide Study of the Prevalence of the Metabolic Syndrome and Optimal Cutoff Points of Waist Circumference in the Middle East. Diabetes Care (2009) 32(6):1092–7. doi:10.2337/dc08-1800
45. Balhara, YPS. Tobacco and Metabolic Syndrome. Indian J Endocrinol Metab (2012) 16(1):81–7. doi:10.4103/2230-8210.91197
46. Coambs, RB, Li, S, and Kozlowski, LT. Age Interacts With Heaviness of Smoking in Predicting Success in Cessation of Smoking. Am J Epidemiol (1992) 135(3):240–6. doi:10.1093/oxfordjournals.aje.a116277
47. Canoy, D, Wareham, N, Luben, R, Welch, A, Bingham, S, Day, N, et al. Cigarette Smoking and Fat Distribution in 21, 828 British Men and Women: A Population-Based Study. Obes Res (2005) 13(8):1466–75. doi:10.1038/oby.2005.177
48. Tonstad, S, and Svendsen, M. Premature Coronary Heart Disease, Cigarette Smoking, and the Metabolic Syndrome. Am J Cardiol (2005) 96(12):1681–5. doi:10.1016/j.amjcard.2005.07.083
49. Alkeilani, A, Khalil, A, Azzan, A, Al-Khal, N, Al-Nabit, N, Talab, O, et al. Association Between Waterpipe Smoking and Obesity:Population-Based Study in Qatar. Tob Induc Dis (2022) 20(January):06–9. doi:10.18332/tid/143878
50. Ward, KD, Ahn, S, Mzayek, F, Al Ali, R, Rastam, S, Asfar, T, et al. The Relationship Between Waterpipe Smoking and Body Weight: Population-Based Findings From Syria. Nicotine Tob Res (2015) 17(1):34–40. doi:10.1093/ntr/ntu121
51. Al-Sawalha, NA, Almahmmod, Y, Awawdeh, MS, Alzoubi, KH, and Khabour, OF. Effect of Waterpipe Tobacco Smoke Exposure on the Development of Metabolic Syndrome in Adult Male Rats. PLoS ONE (2020) 15(6):e0234516. doi:10.1371/journal.pone.0234516
52. Qasim, H, Alarabi, AB, Alzoubi, KH, Karim, ZA, Alshbool, FZ, and Khasawneh, FT. The Effects of Hookah/Waterpipe Smoking on General Health and the Cardiovascular System. Environ Health Prev Med (2019) 24(1):58. doi:10.1186/s12199-019-0811-y
53. Alomari, MA, and Al-Sheyab, NA. Impact of Waterpipe Smoking on Blood Pressure and Heart Rate Among Adolescents: The Irbid-TRY. J Subst Use (2018) 23(3):280–5. doi:10.1080/14659891.2017.1394379
54. Alomari, MA, Al-Sheyab, NA, and Mokdad, AH. Gender-Specific Blood Pressure and Heart Rate Differences in Adolescents Smoking Cigarettes, Waterpipes or Both. Subst Use Misuse (2020) 55(2):296–303. doi:10.1080/10826084.2019.1666146
55. Malekzadeh, MM, Etemadi, A, Kamangar, F, Khademi, H, Golozar, A, Islami, F, et al. Prevalence, Awareness and Risk Factors of Hypertension in a Large Cohort of Iranian Adult Population. J Hypertens (2013) 31(7):1364–71. doi:10.1097/HJH.0b013e3283613053
56. Gee, ME, Bienek, A, Campbell, NRC, Bancej, CM, Robitaille, C, Kaczorowski, J, et al. Prevalence of, and Barriers to, Preventive Lifestyle Behaviors in Hypertension (From a National Survey of Canadians With Hypertension). Am J Cardiol (2012) 109(4):570–5. doi:10.1016/j.amjcard.2011.09.051
57. Rezaei, N, Ahmadi, N, Shams Beyranvand, M, Hasan, M, Gohari, K, Yoosefi, M, et al. Alcohol Consumption and Related Disorders in Iran: Results From the National Surveillance of Non-Communicable Diseases’ Survey (STEPs) 2016. PLOS Glob Public Health (2022) 2(11):e0000107. doi:10.1371/journal.pgph.0000107
Keywords: waterpipe, metabolic syndrome, Iran, cross-sectional, prospective
Citation: Sadeghi Y, Naghash M, Poustchi H, Alvand S, Gandomkar A, Molavi Vardanjani H, Malekzadeh F, Boffetta P, Abnet CC, Freedman ND, Malekzadeh R and Etemadi A (2024) Prevalence and Incidence of Metabolic Syndrome and Its Components Among Waterpipe Users. Int J Public Health 69:1607156. doi: 10.3389/ijph.2024.1607156
Received: 08 February 2024; Accepted: 26 June 2024;
Published: 11 July 2024.
Edited by:
Robert Wellman, UMass Chan Medical School, United StatesReviewed by:
Doo Woong Lee, Massachusetts General Hospital and Harvard Medical School, United StatesOne reviewer who chose to remain anonymous
Copyright © 2024 Sadeghi, Naghash, Poustchi, Alvand, Gandomkar, Molavi Vardanjani, Malekzadeh, Boffetta, Abnet, Freedman, Malekzadeh and Etemadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yasaman Sadeghi, eWFzYW1hbnNhZGVnaGk0OUBnbWFpbC5jb20=; Reza Malekzadeh, ZHIucmV6YS5tYWxla3phZGVoQGdtYWlsLmNvbQ==; Arash Etemadi, YXJhc2guZXRlbWFkaUBuaWguZ292
 Yasaman Sadeghi
Yasaman Sadeghi Mahdokht Naghash1
Mahdokht Naghash1