- 1Department of Circulation and Medical Imaging, Norwegian University of Technology and Science (NTNU), Trondheim, Norway
- 2Department of Neuromedicine and Movement Science, Norwegian University of Technology and Science (NTNU), Trondheim, Norway
Objectives: Representativeness in physical activity randomised controlled trials (RCT) in breast cancer patients is essential to analyses of feasibility and validity considering privileged- social groups. A step-by-step exclusion of less privileged groups through the trial process could reinforce health inequality. This study aimed at examining representativeness in breast cancer (BC) physical activity trials, investigate associations between socio-economic status (SES) and intervention adherence, and explore associations between representativeness and the relationship between SES and intervention adherence.
Methods: Systematic, computerised searches were performed in PubMed, CINAHL, AMED, EMBASE and PsycINFO. Additional citation-based searches retrieved 37 articles. Distributions of education level, ethnicity, and marital status in study samples were compared to national populations data to estimate representativeness in less privileged groups.
Results: A preponderance of studies favoured educated, married and white patients. Only six studies reported SES-adherence associations, hampering conclusions on this relationship and possible associations between representativeness and an SES-adherence relationship.
Conclusion: Less educated, unmarried and non-white individuals may be underrepresented in BC physical activity RCTs, while SES-adherence associations in such trials are inconclusive. Unintentional social misrepresentations may indicate that disguised inequity warrants revived attention.
Introduction
Clinical trials in breast cancer (BC) research have contributed significantly to improved treatments, health-related quality of life, and survival probability for BC patients [1]. For example, numerous clinical physical activity (PA) trials have been conducted. Nevertheless, faced with the reality of social health inequalities [2, 3], it is essential to ensure that the results of these trials benefit all, not exclusively the most privileged. Previous analyses have concluded that study samples only slightly represent real-world patient populations [4] due to participants having better health status, lower age, and a dominant ethnicity. However, research inequity on the part of socioeconomic status (SES)-groups has been scarcely investigated. As PA appears to improve physical fitness [5–8], fatigue [5, 6, 9, 10], and physical functioning [5, 9, 11] in BC patients and survivors, knowledge about representativeness across SES and privilege in randomised controlled trials (RCT) with PA is warranted.
Privilege refers to (often unrecognised) advantages that benefit people belonging to certain groups based on factors like their SES, ethnicity, age, gender identity [12]. High-SES individuals, for example, are considered to have health benefits as a result of their belonging to a privileged social group [13]. SES, is ideally measured as the combination of an individual’s or groups’ education, income and occupation [14] and it is often used to examine social health inequalities [15]. In the case of BC, there is an excess risk associated with increased education, in both men [16] and women [17]. Unmarried women, who face a multitude of hardships and less privilege [18], may have a higher risk of developing BC [19]. Similarly, less privileged non-whites have higher odds of being diagnosed with aggressive BC [20]. However, BC in men and less privileged women is generally diagnosed later, making these cases more severe compared to BC diagnosed in privileged women [21]. Despite higher incidence rates of early BC, higher survival rates and better health-related quality of life after diagnosis [22] are associated with higher education [23]. In young men with invasive BC, overall survival is found to decrease in groups living in ZIP-code areas associated with low SES [24]. Presumably because <1% of all BC incidents occur in men [25], most PA research concern women. Female patients often report weight gain, which is associated with undesirable BC-outcomes [26–28]. Thus, it is often recommended that they pursue regular PA [29]. An inactive lifestyle is suggested to be a significant social determinant of decreased BC survival probability in low-SES groups [30], and according to Boer et al., there is strong evidence that BC recurrence and mortality are strongly associated with leisure-time PA (LTPA) due to the biological mechanisms affected [31].
PA is defined as any bodily movement produced by skeletal muscles that results in energy expenditure [32] and includes the active transport-, leisure time- (including exercise), job-related-, and household PA domains [33]. The relationship between PA and the risk of BC is well documented [34–44] and is evident for LTPA in postmenopausal BC [45, 46]. However, it has been found that different SES-groups in general are inclined to different domains of PA, with high-SES groups being more physically active within the LTPA domain [47]. Comparable results are reported in studies of women with BC [48–51]. Hence, for RCTs with LTPA, there is a risk of selection bias, which is also acknowledged in the Exercise guidelines for cancer survivors: “…the individuals enrolled in studies commonly meet prespecified eligibility criteria (…) and were willing to take part in research. This often results in a sample that is healthier or with higher physical function and exercise motivation that may not fully generalize to the broader population of cancer survivors.” [52].
Moreover, if there are SES-differences in PA among women with BC in general, participation and adherence to PA trials may differ accordingly. Studies have shown different PA barriers across SES [53, 54], partly explaining variances in activity levels [55–59]. If low-SES individuals perform less LTPA than high-SES individuals [47], the former may be prone to lower participation and adherence rates in exercise RCTs. In addition to findings of better exercise trial adherence in high-SES groups [60, 61], less privileged individuals less frequently volunteer to participate in research [62, 63], due to barriers to access [62], lower health literacy and negative attitudes towards research, additional costs, or disease status [64]. These findings may imply that RCTs with which a privileged group is more familiar and motivated for the provided intervention (i.e., LTPA/exercise) run higher risks of selection bias than RCTs for which compliance is less affected by social grouping.
Hence, the external validity of LTPA trials for BC patients may be impaired, albeit unintendedly, by a stepwise exclusion of less privileged patients; they appear to be recruited and participate less frequently, and may also have lower adherence rates [65]. The general view is that RCT samples must be homogeneous to gain internal validity and reliable results [66, 67]. Simultaneously, the declarations of Helsinki [68], legitimately and necessarily, prevents researchers from obliging participation for the purpose of representativeness. Consequently, social biases may be fortified [64, 65].
The credibility and success of medical progress depend on transparent reporting [69]. Thus, the CONSORT guidelines developed to improve RCT-articles [70]. Nevertheless, participant attributes, such as SES-indicators, are seldomly reported [71, 72], impeding successful assessments of equitable research [72]. A reasonable alternative may be to employ the available variables that indicate, or strongly correlate with, privilege and SES. Previous reviews of PA-SES associations have found that education is the most reported SES-indicator [47, 73, 74]. Furthermore, frequently reported characteristics, such as ethnicity and marital status, both reflect social privilege and correlate with SES, thus they may be considered fair representations of privilege in the absence of precise SES-indicators.
The objective of this systematic review was to study previous PA RCTs on BC patients with the intention of examining a) SES-related information and representativeness, b) associations between SES-related indicators and PA intervention adherence, and c) associations between representativeness and reported relationships between SES and intervention adherences. There are, of course, real obstacles to recruting less privileged patients, so non-representative samples should not be seen as researchers’ unwillingness or lack of effort to include the less privileged. The overall aim of the study was not to evaluate single studies conducted for purposes other than representativeness per se, but rather to highlight disguised patterns across comparable studies [75]. Furthermore, the aim was not to provide meta-analyses and precise sample- vs real-world ratios; however, the study may render a departure point for improving representativeness on the part of less privileged groups.
Methods
Systematic, computerised searches were conducted in the PubMed, CINAHL, AMED, EMBASE and PsycINFO databases. The first round followed a traditional method [76]; we specified a search query by a set and combinations of words, and all publications indexed in the databases that contained those words were returned. “Compliance,” “persistence,” “fidelity,” “maintenance” and “concordance” were used as synonyms for the variable “adherence.” Similarly, “physical,” “exercise,” “fitness,” “sport” and “training” were used as synonyms, in addition to exercise-specific terms, such as “dance,” “swim,” “walk” and “yoga,” to cover any type of PA intervention. An asterisk (*) was attached to the roots of the words to broaden the search, and to retrieve variations on these terms. Because the terms are used interchangeably in the literature, both “patients” and “survivors” were used to cover relevant study samples. As recommended by Bramer et al. [77]. Boolean combinations were used to construct suitable search queries in combination with the basic term “breast cancer” (see Figure 1). There were no search limits as to publication year.
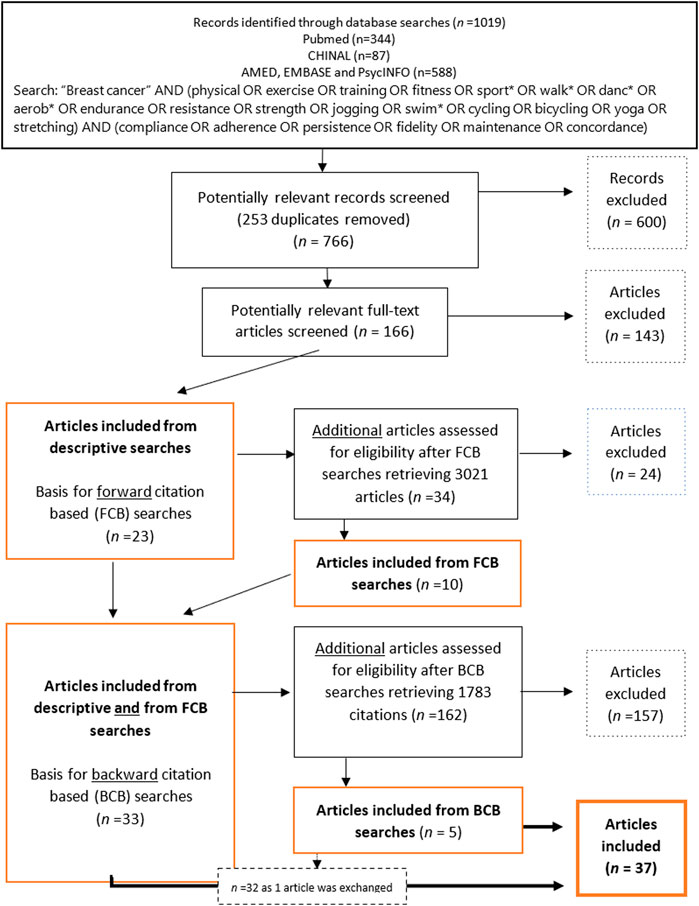
Figure 1. Flow chart of inclusion and exclusion of articles through descriptive- and citation-based searches (Global, 2000–2020).
The following criteria guided the inclusion and exclusion process, defining the final dataset:
Inclusion Criteria
a) Empirical studies
b) …reporting RCTs including a PA intervention.
c) …including individuals undergoing BC treatment within one-year postdiagnosis.
d) …reporting adherence to the PA intervention.
e) …presenting a comprehensible calculation of adherence.
f) …written in English.
Exclusion Criteria
a) Reviews, theoretical and descriptive papers, books, theses, letters to editor or editorials.
Articles:
b) …reporting on multiple cancer diagnoses (even if BC was included).
c) …not reporting any SES-related characteristics of the sample … written in any non-English language.
The descriptive searches retrieved 1,019 articles. After excluding immediate duplicates, 766 articles were manually examined and deemed potentially relevant based on a screening of titles and abstracts. This left, 166 potentially relevant articles that were examined to establish their eligibility according to the criteria. An algorithm for all variables was implemented using Python3 to ensure the eligibility of the articles selected. In total, 143 articles were excluded for a total of 23 remaining articles (Figure 1).
To retrieve all possible relevant studies, thus reducing the risk of missing information [78], citation-based searches (described by Hu et al. [79], but implemented in Rysstad and Pedersen [80] and Darvik et al. [81]) were performed based on the 23 articles found in the descriptive searches. A forward citation-based (FCB) search of articles that cited the articles included in the descriptive searches was first reviewed for eligibility, and 34 potentially relevant articles were identified, 24 of which were excluded. A backwards citation-based (BCB) search, where the reference lists in the 33 articles already included in the material were scanned for eligible studies, retrieved 5 articles of which one replaced a previously included article, following the inclusion criteria.
In cases in which an article reported on two different parent trials, the article was included on the condition that at least one of the parent studies met inclusion criteria c. If two articles were reporting on the same parent RCT sample and overlapped in their data, the earliest article was included. If, however, only one of them reported adherence rates, the article omitting adherence rates was excluded. Nevertheless, the included article had to report on SES-related patient characteristics. In the subsequent step of the inclusion/exclusion process, only articles reporting the samples’ SES distributions were included for further analyses.
The second author performed the database searches but conferred with the first author to discuss any cases of doubt about the potentially relevant articles returned from the query.
Data Extraction and Analysis
The following variables were extracted from the articles: sample descriptions, intervention designs, aims of the studies, measures of SES or privilege, their distributions, and adherence calculations and -rates. For studies that investigated the associations between adherence and SES-related factors, the reported results were registered.
The SES-related distribution in the sample was compared with the corresponding distribution within the country in which the study was conducted, at the matching time of publication. Because adherence to intervention protocols was embedded in our study objectives, only intervention group characteristics were analysed. Due to third-party researchers’ limited access to patient population data, SES-related distribution in a country population, in the corresponding age groups, was used as a proxy for the patient population. For the education variable, the percentage of the study sample holding any tertiary education (no degree required) was compared with the percentage of the country population holding the same educational level (i.e., all formal postsecondary education, including public and private universities, colleges, technical training institutes, and vocational schools [82]), using public statistics for reference [83–86] (see Supplementary Files S2, S3 for details). Because the final data set included articles about women exclusively, all statistics retrieved and analysed, were relevant to women.
Similarly, the proportion of married women in the study samples and in the associated country populations was compared using United Nations World Marriage Data (age 45–49) [87] and a corresponding analysis for ethnicity based on national censuses [88–92]. Frequencies in the white alone and non-indigenous and non-visible minority categories from accessible censuses were used to estimate figures for intercensal years by interpolation. The relative differences between the study samples and the associated countries were calculated as follows:
Studies reporting on the association between SES-related factors and adherence to the PA intervention were further explored, and variations in adherence calculations were examined.
Results
The preliminary screening excluded 20%–25% of all potentially relevant articles due to a lack of information about SES-related characteristics. The subsequent selection process, following descriptive, FBC- and BCB-searches, retrieved 37 eligible articles (Figure 1).
A total of 28 (76%) of the included studies were published between 2010 and 2020, and the oldest article was published in 2002 [93]. Most studies (81%) were conducted in Western countries: 18 in the United States, 5 in Canada, two in the Netherlands, and one each in Spain, France, the United Kingdom, Denmark and Sweden. Six (16%) studies were Asian, including one Indian, two Chinese and three Taiwanese studies. In addition, one Brazilian study was included. The (full) sample sizes ranged from 14 [94] to 301 [95], including 11 studies with <50 participants, 15 studies with 50–96 participants, and 11 studies with 100–301 participants. The mean age ranged from 42.1 to 63.2 years (total mean = 51 years) (Table 1). All included studies were of female BC patients.
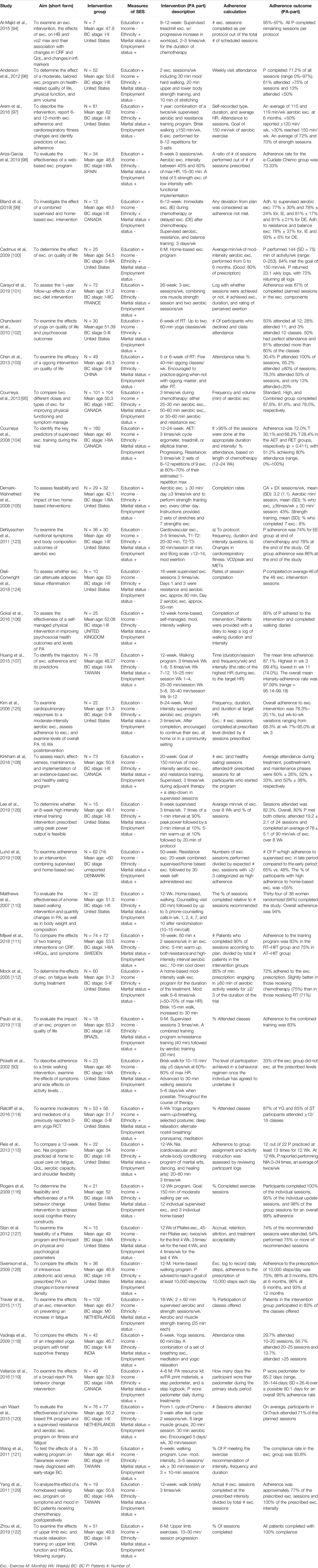
Table 1. Study aim, sample, SES and intervention descriptions, adherence formula, and adherence outcome in included studies. (Global, 2000–2020).
Education was the most frequently used indicator of privilege and was found in 30 (81%) of the articles [93–122] (Table 1). Marital status was reported in 28 (76%) [93–95, 97–99, 101–109, 111–115, 122], ethnicity in 22 articles (59%) [93–95, 102, 105, 108, 110, 112, 114–116, 119, 123–128] employment status in 25 articles (68%) [93–95, 98, 101, 102, 104, 106–111, 113–115, 118, 119], and income in eight articles (22%) [95, 96, 103, 104, 114, 116, 122, 123]. Two articles [95, 114] reported five indicators, and four [124, 126, 127, 129] reported one. Ethnicity was reported in all 18 USA studies, as well as in four of the five Canadian studies, but it was not reported in the remaining studies. For the rest of the indicators, no geographical patterns were identified.
There was no uniform definition of adherence although the most common calculation was attendance rates relative to total, or weekly, prescribed, or possible, PA sessions. The mean adherence rate for these studies was 78.9% (30.4%–100%). All studies reported on patients diagnosed with BC in stages 0–III.*. Most studies reported the feasibility or effect of a PA intervention on patients undergoing radiation-, chemotherapy, or both.
The PA-interventions were designed differently in terms of PA type (e.g., walking, exercise including endurance and strength, yoga, aerobics, martial arts, dancing, cycling, qigong, balance training, Pilates), intensity (e.g., according to Ainsworth et al. [130], yoga is performed at 2.5 METs, whereas bicycling could require 4–16 METs), duration (10–60 min/session, 6 weeks–12 months/intervention) and frequency (voluntarily–5 bouts/week).
Privileged Groups and Adherence to PA Interventions
Some articles were feasibility studies centred on adherence; others reported adherence as a sub-analysis. Six studies reported on the relationship between SES-related factors and adherence to or completion of the intervention [97, 104, 107, 109, 111, 114]. However, two of these studies reported no significant difference in participant characteristics between withdrawers and completers [111] or between those with 75% attendance and other participants [114], without specified SES-indicators. Three of these six studies indicated a positive, however weak, association between adherence to an aerobic- and strength training protocol and educational level [97, 104, 109]. One study [107] noted that employment status was associated with adherence measured as intensity, but not when adherence was assessed in terms of exercise time. No differences in adherence across educational levels were reported in this study.
Representativeness in terms of Research Participants’ Education, Ethnicity and Marital Status
Figure 2 displays the sample-country differences in educational level. Eight studies had a lower proportion of individuals with higher education compared to the country population [97–99, 101, 106, 107, 114, 121], whereas 21 studies showed the opposite trend. In 11 of these studies, the proportion of participants who had tertiary education in the population was >25% lower compared to the sample [94, 96, 100, 102–105, 109, 111, 120, 122]. Four studies had a difference ≤10% [93, 95, 108, 115] (Figure 2; Table 1 in Supplementary Files S1–S3).
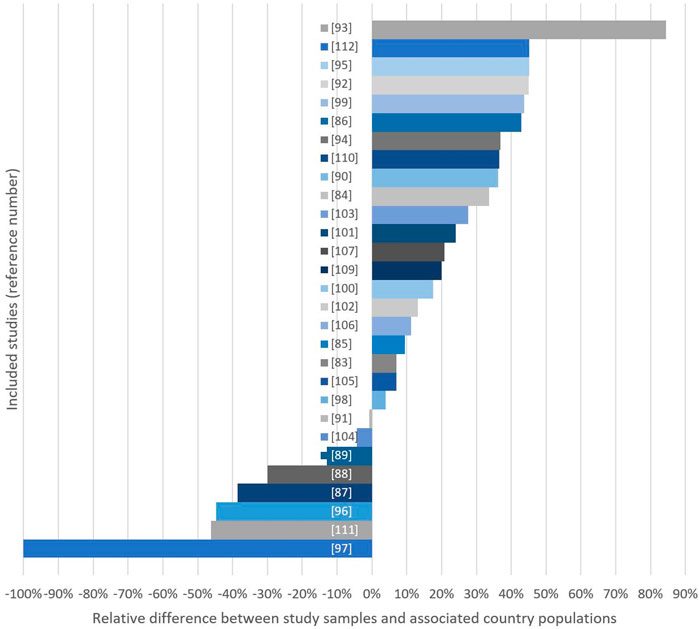
Figure 2. Relative differences (%) between study samples and country populations considering either mean years of education (*) or % of women with >1 year of high education in study sample. Bars extending the right side indicate studies with high-education group overrepresentation (Global, 2000–2020).
The mean proportion of married women in the relevant samples was 71.9% (SD = 11.68), while the corresponding number was 73.9 (SD = 7.32) for the country populations (Figure 3; Table 2 in Supplementary File S1). Sixteen of the studies using marital status reported their results on samples with a lower proportion of married women compared to the country population [93, 95, 97, 98, 103, 104, 107, 108, 111–115, 121, 122, 125], while 11 examined samples with a higher proportion of married women compared to the country population [94, 99, 101, 102, 106, 109, 117–119, 123, 131]. One study [105] showed no difference (Tables 2, 3 in Supplementary File S1).
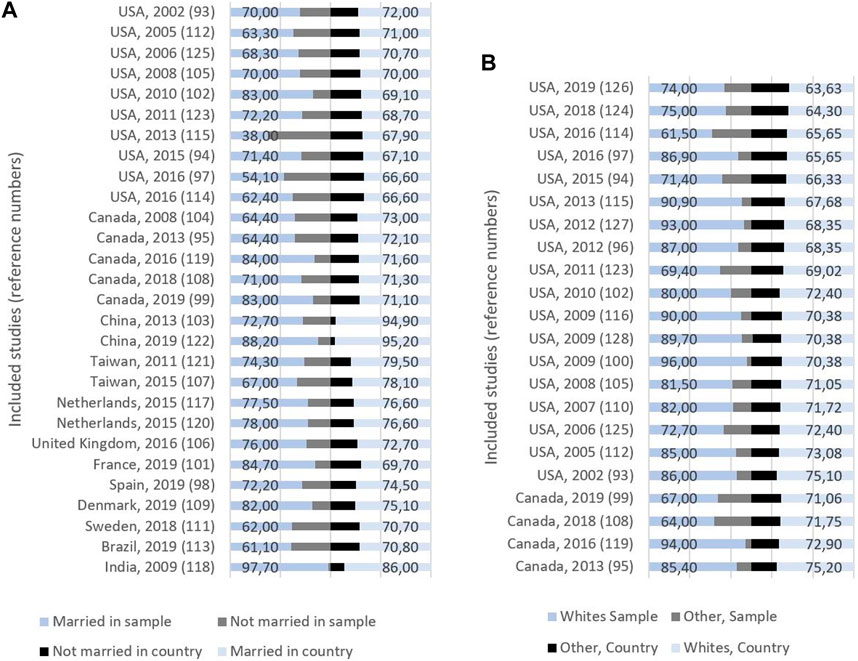
Figure 3. (A) Study origin, publication year, and proportion (%) of married and non-married women in sample (left) and country (right). (B) Study origin, publication year, and proportion (%) of whites and other ethnicities in sample (left) and country (right) (Global, 2000–2020).
In the 22 studies that reported ethnicity, the mean proportion of whites was 81%: 77.6% Canadian studies, and 81.8% American studies (Figure 3; Table 3 in Supplementary File S1). Three of these studies reported their results on samples with a lower proportion of whites compared with the country population, while the remaining 19 studies had samples with a higher proportion of whites. There was a difference between samples and countries of 10%–25% in 15 of these studies (Table 3 in Supplementary File S1).
Representativeness and Adherence
There was no clear relationship between the registered representativeness and the associations between indicators of SES and adherence. Three of the six mentioned studies reporting such associations [97, 107, 114] had samples with a lower proportion of high-SES BC patients compared to their country populations, while the other three [104, 109, 111] had samples with a higher proportion of high-SES patients.
Discussion
To our knowledge, this is the first systematic review to report the social representativeness of RCTs examining the effects of PA on BC-related outcomes. In the included dataset, there was a preponderance of studies overrepresenting groups associated with higher privilege, supporting previous results indicating social bias [132]. Misrepresentations were found across different indicators of privilege, likely because different indicators tend to interrelate [133]. Our findings coincide with earlier observations that low-SES patients are less inclined to participate in research than high-SES patients [62, 63], as well as with previous findings of obstacles to recruiting less privileged populations to cancer RCTs [64].
The most pronounced findings were based on studies that reported participants’ education. However, in six studies that did not record education but rather recorded ethnicity/race, as in the total dataset, there was a preponderance of white participants. In agreement with previous reports [62, 132, 134], this was interpreted as recruitment bias, although these analyses were based on less accurate data compared to the analyses on education. All 22 studies reporting ethnicity were from the USA and Canada, where the ethnic distribution varies over time and space, particularly in the United States [91]. Although education and income relate to ethnicity [135], our results on the representativeness of ethnic groups are unclear due to inaccurate data and increased confusion among the populations regarding ethnic categories. In general, it may appear inappropriate to use the category white as an exclusive indicator of social privilege in global comparative analyses such as the current study. Nonetheless, in the overall discussion of representativeness in RCTs, research favouring certain ethnicities over others should not be supported.
Representativeness was further assessed by marital status, and the mean difference between the study samples and the associated country populations was small. However, the differences ranged from −78% to 17.7%, and only 9 studies differed less than 5% from the country population. This means that although marital status is considered a measure of whether people have the social and financial benefits of being coupled, it could affect the possibility of being included in a PA trial.
What is considered an acceptable level of representativeness in RCT samples is difficult to decide. Recruitment issues may lead to sample biases, as patient populations often differ across social groups due to their diagnoses or hospital characteristics. However, the body of articles from the current dataset that employed education as the SES-indicator included eight studies with (“negative” differences from the country population and 22 studies with “positive” differences (including 11 samples with >25% difference from the country population), which are significant findings. The fact that studies with a negative deviation from the country’s corresponding distribution in this subgroup were published after 2015 could be an effect of the CONSORT guidelines [70] and the Belmont report [136], which emphasised the implications of selection biases. However, our screening and analyses clearly show that there is still a potential for improvement among researchers in their attention to social distribution in samples.
Our results may have been more valid if baseline characteristics were compared with a real-world patient population and a patient sample (see [4]). However, such analyses require that third-party researchers also have access to exact data about the patient populations within each region, at the precise time of the studies, in addition to which patients are eligible according to each of the RCT criteria. The primary aim of the present study was to revive the question of external validity and feasibility from a perspective of social health inequalities in PA research. Therefore, status distribution in the respective age groups within the country populations served as an acceptable proxy for the distribution in the patient population.
Another objection may be that, for a patient to be considered eligible for a PA RCT, their cancer must be at a stage compatible with physical exertion and that more advanced BCs are associated with lower privilege [137]. Hence, RCTs with exercise often include patients with higher SES (education) because they have less advanced BC. However, this claim confirms the importance of putting focus on representativeness in RCTs targeting an already socially skewed patient population; it is important to be even more aware of sample representativeness to avoid excessive bias by favouring the already privileged. Likewise, employing already skewed patient populations as a reference could promote further social inequalities in health. Nonetheless, if the reference ratio for assessing representativeness was 1.22 [17], it would be possible to accept 22% more high-education patients before claiming misrepresentation of less privileged groups. However, according to our results, based on patients’ educational levels, approximately 45% of the included studies would still be considered biased in favour of the most privileged patients.
SES and PA Intervention Adherence
A proper body of articles with analyses of the association between SES-related characteristics and adherence was expected, and also that these articles would provide a basis for a synthesised examination of this relationship. However, the fact that only 6 studies investigated this association suggests that this question is perceived to be of little relevance in the field of BC and PA research. A previous review on the representativeness of RCTs reported no socioeconomic misrepresentation in oncology studies [4] however, our interpretation is supported by the fact that the review included only articles that provided a representativeness analysis, which the oncology articles had omitted in the case of SES.
No clear trend of associations between SES-related factors and adherence was seen across the six aforementioned studies, and the small number of studies and the differences between them hampered a clear conclusion. Previous studies have argued that poor representativeness may explain a lack of associations between SES and adherence rates [138, 139]. In the current data set, there was no clear association between representativeness and SES-adherence associations. However, the small number of relevant articles formed an overly scarce base for reliable and conclusive analyses of this research question.
A tendency for studies that found a positive relationship between SES-related factors and adherence to report on longer interventions and to calculate adherence in terms of rates of attendance was observed. Three studies reported that the adherence rates decreased over time [97, 104, 107]. Previous results support the interpretation that time is a barrier to participation in PA interventions [53, 140]. Strazdin et al. have substantiated how availability of time may have a larger impact on low-SES women in general [141, 142]. Hence, the limited availability of time over a prolonged period may partly explain why less privileged patients have poorer adherence in such trials than their privileged counterparts.
Reasons for refusing to participate, or reasons for withdrawing, were not registered in our study. We should not ignore the fact that researchers experience real obstacles in recruiting of less privileged patients, so non-representative samples should not be seen as unwillingness or lack of effort to include the less privileged. There are reasons to believe that reasons for refusal to participate in the articles included coincide with the most common barriers previously reported [10, 64, 131]. However, although participation is voluntary and individual motivation or barriers are relevant, the adherence rates and the current social group distribution of patients in RCTs with PA may also be affected by the intervention design and the inclusion criteria defined by the researchers of each individual study [64].
All the included studies reported LTPA interventions. Considering that low-SES groups engage less in LTPA [47], it is reasonable to expect that low-SES patients were less inclined to participate in, and complete, such RCTs. Hence, a preponderance of RCTs employing exercise interventions could be perceived as being in favour of privileged patients. The searches of the current study did not, however, include search terms covering other PA domains and may thereby have strengthened the impression that LTPA is the prevailing protocol in RCTs of PA in BC patients. However, although many patients return to work during their first-year post diagnosis, RCTs designed for other PA-domains would not be verifiable as the patients’ level of PA would differ too much to control. In addition, the control group would have to be inactive within these PA-domains, which would be a requirement almost impossible to implement. The probability that many published RCTs of other types of PA exist, thus altering our results, is therefore small.
Consequences of Enhanced Selection Bias in Favour of Privileged Patients
Provided that our results reflect reality as they are based on synthesised data from a large set of systematically selected articles, social equity is a challenge to PA trials in BC patients. A stepwise, albeit unintended, social exclusion process causes attrition bias and a successive decrease in external validity, (i.e., the results are even less valid for individuals in other social groups than those who have completed the intervention when the intervention is finalised) [65]. Thus, PA-treatment is also less adaptable to patients in the same groups as those who withdraw. Drawing on the fundamental cause theory [143], these mechanisms show how affluent people could benefit more, in this case, from PA research, because studies serve the interests of these groups more than they care for all SES-groups. In addition, insignificant results from sub-analyses of social group-differences may be misinterpreted because the distribution of social groups in the samples does not mirror real life. This may disguise the possible fact that although all patients, by policy, should have equal access to health services, PA treatment interventions may not be suitable for all. An “inverse PA research law” analogous to the “inverse healthcare law” [144] describes how PA research interventions, initiated with the intention to treat a patient population, regardless of social status, nevertheless attend to privileged groups more than the less privileged who initially are prone to poorer health and thus more entitled to clinical research.
Strengths and Limitations
The major strength of this research is the thorough and systematic search of eligible studies, including traditional, and both BCB and FCB searches. To avoid our searches and not be included, an article would have to be published in a journal that is not indexed in any of the chosen databases, not be identified by the search terms, not be included in either the reference lists of the articles included from the descriptive searches or not have cited the articles included from the descriptive searches. It is possible that some articles were lost. However, it is less likely that any articles not included would be systematically more representative compared with the articles included.
The sample sizes in one-third of the studies were <50, seemingly decreasing the validity of our conclusions. However, rather than being a shortcoming of our study, the limitation is applicable to each RCT because the small samples, among other factors, hamper representativeness analyses such as ours. Hence, our analyses were based on the publication reality, whatever flawed original data materials.
SES and social privilege classifications vary across cultures, research fields, and public statistics, and they may cause imprecise standards for comparisons between studies of different origins. Nevertheless, our results present the big picture of misrepresentation across social groups with a precision sufficient to boost the debate within the scientific community.
Conclusion
Less educated, unmarried and non--white individuals may be underrepresented in BC PA RCTs, and SES-adherence associations in such trials are inconclusive. Unintentional social misrepresentation may create disguised inequity, warranting revived attention to this issue.
The current study provides a departure point for intensified attention to representativeness in RCTs. It should act as motivation both for seeking improved external validity, and for reconsidering whether LTPA in BC treatment is suitable for all.
Author Contributions
RS and MD made substantial contributions to the study: RS conceived the idea of the study, including its design, methodology and analysis. MD and RS performed descriptive- and FC searches, and BC searches, respectively, and the subsequent inclusion/exclusion of articles. RS and MD performed the analyses. RS drafted the manuscript. RS and MD reviewed and edited the manuscript and approved the final version. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2024.1607002/full#supplementary-material
References
1. Wickerham, DL, O'Connell, MJ, Costantino, JP, Cronin, WM, Paik, S, and Geyer, CE. The Half century of Clinical Trials of the National Surgical Adjuvant Breast and Bowel Project. In: Seminars in Oncology. Amsterdam, Netherlands: Elsevier (2008).
2. Marmot, M, and Bell, R. Social Inequalities in Health: A Proper Concern of Epidemiology. Ann Epidemiol (2016) 26(4):238–40. doi:10.1016/j.annepidem.2016.02.003
3. Mackenbach, JP. Persistence of Social Inequalities in Modern Welfare States: Explanation of a Paradox. Scand J Public Health (2017) 45(2):113–20. doi:10.1177/1403494816683878
4. Kennedy-Martin, T, Curtis, S, Faries, D, Robinson, S, and Johnston, J. A Literature Review on the Representativeness of Randomized Controlled Trial Samples and Implications for the External Validity of Trial Results. Trials (2015) 16(1):495–14. doi:10.1186/s13063-015-1023-4
5. Dantzer, R, Meagher, MW, and Cleeland, CS. Translational Approaches to Treatment-Induced Symptoms in Cancer Patients. Nat Rev Clin Oncol (2012) 9(7):414–26. doi:10.1038/nrclinonc.2012.88
6. Furmaniak, AC, Menig, M, and Markes, MH. Exercise for Women Receiving Adjuvant Therapy for Breast Cancer. Cochrane Database Syst Rev (2016) 9:CD005001. doi:10.1002/14651858.CD005001.pub3
7. Irwin, ML, Crumley, D, McTiernan, A, Bernstein, L, Baumgartner, R, Gilliland, FD, et al. Physical Activity Levels before and after a Diagnosis of Breast Carcinoma: The Health, Eating, Activity, and Lifestyle (HEAL) Study. Cancer Interdiscip Int J Am Cancer Soc (2003) 97(7):1746–57. doi:10.1002/cncr.11227
8. Protani, M, Coory, M, and Martin, JH. Effect of Obesity on Survival of Women with Breast Cancer: Systematic Review and Meta-Analysis. Breast Cancer Res Treat (2010) 123(3):627–35. doi:10.1007/s10549-010-0990-0
9. Husebo, AML, Karlsen, B, Allan, H, Soreide, JA, and Bru, E. Factors Perceived to Influence Exercise Adherence in Women with Breast Cancer Participating in an Exercise Programme during Adjuvant Chemotherapy: A Focus Group Study. J Clin Nurs (2015) 24(3-4):500–10. doi:10.1111/jocn.12633
10. Maddocks, M, Mockett, S, and Wilcock, A. Is Exercise an Acceptable and Practical Therapy for People with or Cured of Cancer? A Systematic Review. Cancer Treat Rev (2009) 35(4):383–90. doi:10.1016/j.ctrv.2008.11.008
11. Bhute, VJ, Ma, Y, Bao, X, and Palecek, SP. The Poly (ADP-Ribose) Polymerase Inhibitor Veliparib and Radiation Cause Significant Cell Line Dependent Metabolic Changes in Breast Cancer Cells. Sci Rep-uk (2016) 6(1):36061. doi:10.1038/srep36061
12. Black, LL, and Stone, D. Expanding the Definition of Privilege: The Concept of Social Privilege. J Multicultural Couns Dev (2005) 33(4):243–55. doi:10.1002/j.2161-1912.2005.tb00020.x
13. Reynolds, MM. Health Power Resources Theory: A Relational Approach to the Study of Health Inequalities. J Health Soc Behav (2021) 62(4):493–511. doi:10.1177/00221465211025963
14. Lampert, T, Hoebel, J, Kuntz, B, Müters, S, and Kroll, LE. Socioeconomic Status and Subjective Social Status Measurement in KiGGS Wave 2. J Health Monit (2018) 3(1):108–25. doi:10.17886/RKI-GBE-2018-033
15. Braveman, PA, Cubbin, C, Egerter, S, Chideya, S, Marchi, KS, Metzler, M, et al. Socioeconomic Status in Health Research: One Size Does Not Fit All. JAMA (2005) 294(22):2879–88. doi:10.1001/jama.294.22.2879
16. D'Avanzo, B, and La Vecchia, C. Risk Factors for Male Breast Cancer. Br J Cancer (1995) 71(6):1359–62. doi:10.1038/bjc.1995.264
17. Dong, J-Y, and Qin, L-Q. Education Level and Breast Cancer Incidence: A Meta-Analysis of Cohort Studies. Menopause (2020) 27(1):113–8. doi:10.1097/GME.0000000000001425
18. Kearney, MS. The Two-Parent Privilege: How the Decline in Marriage Has Increased Inequality and Lowered Social Mobility, and what We Can Do about it. Great Britain: Swift Press (2023).
19. Li, M, Han, M, Chen, Z, Tang, Y, Ma, J, Zhang, Z, et al. Does Marital Status Correlate With the Female Breast Cancer Risk? A Systematic Review and Meta-Analysis of Observational Studies. PLoS One (2020) 15(3):e0229899.
20. Sineshaw, HM, Gaudet, M, Ward, EM, Flanders, WD, Desantis, C, Lin, CC, et al. Association of Race/ethnicity, Socioeconomic Status, and Breast Cancer Subtypes in the National Cancer Data Base (2010–2011). Breast Cancer Res Tr (2014) 145(3):753–63. doi:10.1007/s10549-014-2976-9
21. Krieger, N, Wright, E, Chen, JT, Waterman, PD, Huntley, ER, and Arcaya, M. Cancer Stage at Diagnosis, Historical Redlining, and Current Neighborhood Characteristics: Breast, Cervical, Lung, and Colorectal Cancers, Massachusetts, 2001–2015. Am J Epidemiol (2020) 189(10):1065–75. doi:10.1093/aje/kwaa045
22. Klassen, AC, and Smith, KC. The Enduring and Evolving Relationship between Social Class and Breast Cancer burden: A Review of the Literature. Cancer Epidemiol (2011) 35(3):217–34. doi:10.1016/j.canep.2011.02.009
23. Trewin, CB, Johansson, ALV, Hjerkind, KV, Strand, BH, Kiserud, CE, and Ursin, G. Stage-Specific Survival Has Improved for Young Breast Cancer Patients since 2000: But Not Equally. Breast Cancer Res Tr (2020) 182:477–89. doi:10.1007/s10549-020-05698-z
24. Flaherty, DC, Bawa, R, Burton, C, and Goldfarb, M. Breast Cancer in Male Adolescents and Young Adults. Ann Surg Oncol (2017) 24(1):84–90. doi:10.1245/s10434-016-5586-4
25. Siegel, RL, Miller, KD, and Jemal, A. Cancer Statistics, 2018. CA: a Cancer J clinicians (2018) 68(1):7–30. doi:10.3322/caac.21442
26. Patterson, RE, Cadmus, LA, Emond, JA, and Pierce, JP. Physical Activity, Diet, Adiposity and Female Breast Cancer Prognosis: A Review of the Epidemiologic Literature. Maturitas (2010) 66(1):5–15. doi:10.1016/j.maturitas.2010.01.004
27. Nichols, HB, Trentham-Dietz, A, Egan, KM, Titus-Ernstoff, L, Holmes, MD, Bersch, AJ, et al. Body Mass index before and after Breast Cancer Diagnosis: Associations with All-Cause, Breast Cancer, and Cardiovascular Disease Mortality. Cancer Epidemiol Biomarkers Prev (2009) 18(5):1403–9. doi:10.1158/1055-9965.EPI-08-1094
28. Healy, L, Ryan, A, Carroll, P, Ennis, D, Crowley, V, Boyle, T, et al. Metabolic Syndrome, central Obesity and Insulin Resistance Are Associated with Adverse Pathological Features in Postmenopausal Breast Cancer. Clin Oncol-uk (2010) 22(4):281–8. doi:10.1016/j.clon.2010.02.001
29. Rock, CL, Doyle, C, Demark-Wahnefried, W, Meyerhardt, J, Courneya, KS, Schwartz, AL, et al. Nutrition and Physical Activity Guidelines for Cancer Survivors. CA: A Cancer J Clinicians (2012) 62(4):243–74. doi:10.3322/caac.21142
30. Lundqvist, A, Andersson, E, Ahlberg, I, Nilbert, M, and Gerdtham, U Socioeconomic Inequalities in Breast Cancer Incidence and Mortality in Europe-A Systematic Review and Meta-Analysis. Eur J Public Health (2016) 26(5):804–13. doi:10.1093/eurpub/ckw070
31. de Boer, MC, Wörner, EA, Verlaan, D, and van Leeuwen, PA. The Mechanisms and Effects of Physical Activity on Breast Cancer. Clin Breast Cancer (2017) 17(4):272–8. doi:10.1016/j.clbc.2017.01.006
32. Caspersen, CJPK, and Christenson, GM. Physical Activity, Exercise, and Physical Fitness: Definitions and Distinctions for Health-Related Research. Public Health Rep (1985) 100(2):126–31.
33. Warren, JM, Ekelund, U, Besson, H, Mezzani, A, Geladas, N, Vanhees, L, et al. Assessment of Physical Activity – A Review of Methodologies with Reference to Epidemiological Research: A Report of the Exercise Physiology Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil (2010) 17(2):127–39. doi:10.1097/HJR.0b013e32832ed875
34. Dorgan, JF, Brown, C, Barrett, M, Splansky, GL, Kreger, BE, D'Agostino, RB, et al. Physical Activity and Risk of Breast Cancer in the Framingham Heart Study. Am J Epidemiol (1994) 139(7):662–9. doi:10.1093/oxfordjournals.aje.a117056
35. Fraser, GE, and Shavlik, D. Risk Factors, Lifetime Risk, and Age at Onset of Breast Cancer. Ann Epidemiol (1997) 7(6):375–82. doi:10.1016/s1047-2797(97)00042-2
36. Cerhan, JR, Chiu, BC, Wallace, RB, Lemke, JH, Lynch, CF, Torner, JC, et al. Physical Activity, Physical Function, and the Risk of Breast Cancer in a Prospective Study Among Elderly Women. J Gerontol Ser A: Biol Sci Med Sci (1998) 53(4):M251–6. doi:10.1093/gerona/53a.4.m251
37. Rockhill, B, Willett, WC, Hunter, DJ, Manson, JE, Hankinson, SE, and Colditz, GA. A Prospective Study of Recreational Physical Activity and Breast Cancer Risk. Arch Intern Med (1999) 159(19):2290–6. doi:10.1001/archinte.159.19.2290
38. Thune, I, Brenn, T, Lund, E, and Gaard, M. Physical Activity and the Risk of Breast Cancer. N Engl J Med (1997) 336(18):1269–75. doi:10.1056/NEJM199705013361801
39. Mittendorf, R. Strenuous Physical Activity in Young Adulthood and Risk of Breast Cancer (United States) (Vol 6, Pg 352, 1996). Cancer Cause Control (1996) 7(2):291.
40. Bernstein, L, Henderson, BE, Hanisch, R, Sullivan-Halley, J, and Ross, RK. Physical Exercise and Reduced Risk of Breast Cancer in Young Women. J Natl Cancer (1994) 86(18):1403–8. doi:10.1093/jnci/86.18.1403
41. Friedenreich, CM, and Rohan, TE. Physical Activity and Risk of Breast Cancer. Eur J Cancer Prev (1995) 4(2):145–51. doi:10.1097/00008469-199504000-00004
42. McTiernan, A, Stanford, JL, Weiss, NS, Daling, JR, and Voigt, LF. Occurrence of Breast Cancer in Relation to Recreational Exercise in Women Age 50-64 Years. Epidemiology (1996) 7(6):598–604. doi:10.1097/00001648-199611000-00006
43. Chen, CL, White, E, Malone, KE, and Daling, JR. Leisure-Time Physical Activity in Relation to Breast Cancer Among Young Women (Washington, United States). Cancer Causes Control (1997) 8(1):77–84. doi:10.1023/a:1018439306604
44. Gammon, MD, John, EM, and Britton, JA. Recreational and Occupational Physical Activities and Risk of Breast Cancer. J Natl Cancer Inst (1998) 90(2):100–17. doi:10.1093/jnci/90.2.100
45. Monninkhof, EM, Elias, SG, Vlems, FA, van der Tweel, I, Schuit, AJ, Voskuil, DW, et al. Physical Activity and Breast Cancer - A Systematic Review. Epidemiology (2007) 18(1):137–57. doi:10.1097/01.ede.0000251167.75581.98
46. Friedenreich, CM. Physical Activity and Breast Cancer: Review of the Epidemiologic Evidence and Biologic Mechanisms. Recent Results Cancer Res (2011) 188:125–39. doi:10.1007/978-3-642-10858-7_11
47. Stalsberg, R, and Pedersen, AV. Are Differences in Physical Activity across Socioeconomic Groups Associated with Choice of Physical Activity Variables to Report? Int J Environ Res Public Health (2018) 15(5):922. doi:10.3390/ijerph15050922
48. Hong, S, Bardwell, WA, Natarajan, L, Flatt, SW, Rock, CL, Newman, VA, et al. Correlates of Physical Activity Level in Breast Cancer Survivors Participating in the Women's Healthy Eating and Living (WHEL) Study. Breast Cancer Res Tr (2007) 101(2):225–32. doi:10.1007/s10549-006-9284-y
49. Bertram, LA, Stefanick, ML, Saquib, N, Natarajan, L, Patterson, RE, Bardwell, W, et al. Physical Activity, Additional Breast Cancer Events, and Mortality Among Early-Stage Breast Cancer Survivors: Findings from the WHEL Study. Cancer Causes Control (2011) 22(3):427–35. doi:10.1007/s10552-010-9714-3
50. Keegan, TH, Shariff-Marco, S, Sangaramoorthy, M, Koo, J, Hertz, A, Schupp, CW, et al. Neighborhood Influences on Recreational Physical Activity and Survival after Breast Cancer. Cancer Causes Control (2014) 25(10):1295–308. doi:10.1007/s10552-014-0431-1
51. Boyle, T, Vallance, JK, Ransom, EK, and Lynch, BM. How Sedentary and Physically Active Are Breast Cancer Survivors, and Which Population Subgroups Have Higher or Lower Levels of These Behaviors? Support Care Cancer (2016) 24(5):2181–90. doi:10.1007/s00520-015-3011-3
52. Campbell, KL, Winters-Stone, KM, Wiskemann, J, May, AM, Schwartz, AL, Courneya, KS, et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med Sci Sports Exerc (2019) 51(11):2375–90. doi:10.1249/MSS.0000000000002116
53. Oyekanmi, G, and Paxton, RJ. Barriers to Physical Activity Among African American Breast Cancer Survivors. Psycho-Oncology (2014) 23(11):1314–7. doi:10.1002/pon.3527
54. Owusu, C, Antognoli, E, Nock, N, Hergenroeder, P, Austin, K, Bennet, E, et al. Perspective of Older African-American and Non-Hispanic white Breast Cancer Survivors from Diverse Socioeconomic Backgrounds toward Physical Activity: A Qualitative Study. J Geriatr Oncol (2018) 9(3):235–42. doi:10.1016/j.jgo.2017.12.003
55. Grzywacz, JG, and Marks, NF. Social Inequalities and Exercise during Adulthood: Toward an Ecological Perspective. J Health Soc Behav (2001) 42(June):202–20. doi:10.2307/3090178
56. Lee, RE, and Cubbin, C. Neighborhood Context and Youth Cardiovascular Health Behaviors. Am J Public Health (2002) 92(3):428–36. doi:10.2105/ajph.92.3.428
57. Varo, JJ, Martinez-Gonzalez, MA, de Irala-Estevez, J, Kearney, J, Gibney, M, and Martinez, JA. Distribution and Determinants of Sedentary Lifestyles in the European Union. Int J Epidemiol (2003) 32(1):138–46. doi:10.1093/ije/dyg116
58. Jenum, AK, Lorentzen, CAN, and Ommundsen, Y. Targeting Physical Activity in a Low Socioeconomic Status Population: Observations from the Norwegian “Romsås in Motion” Study. Br J Sport Med (2009) 43(1):64–9. doi:10.1136/bjsm.2008.053637
59. Trost, SG, Owen, N, Bauman, AE, Sallis, JF, and Brown, W. Correlates of Adults’ Participation in Physical Activity: Review and Update. Med Sci Sports Exerc (2002) 34(12):1996–2001. doi:10.1097/00005768-200212000-00020
60. Austin, JL, Smith, JE, Gianini, L, and Campos-Melady, M. Attitudinal Familism Predicts Weight Management Adherence in Mexican–American Women. J Behav Med (2013) 36(3):259–69. doi:10.1007/s10865-012-9420-6
61. Lemstra, M, and Rogers, MR. The Importance of Community Consultation and Social Support in Adhering to an Obesity Reduction Program: Results from the Healthy Weights Initiative. Patient Preference and Adherence (2015) 9:1473–80. doi:10.2147/PPA.S91912
62. Sharrocks, K, Spicer, J, Camidge, DR, and Papa, S. The Impact of Socioeconomic Status on Access to Cancer Clinical Trials. Br J Cancer (2014) 111(9):1684–7. doi:10.1038/bjc.2014.108
63. Langhammer, A, Krokstad, S, Romundstad, P, Heggland, J, and Holmen, J. The HUNT Study: Participation Is Associated with Survival and Depends on Socioeconomic Status, Diseases and Symptoms. BMC Med Res Methodol (2012) 12(1):143. doi:10.1186/1471-2288-12-143
64. Ford, JG, Howerton, MW, Lai, GY, Gary, TL, Bolen, S, Gibbons, MC, et al. Barriers to Recruiting Underrepresented Populations to Cancer Clinical Trials: A Systematic Review. Cancer (2008) 112(2):228–42. doi:10.1002/cncr.23157
65. Stalsberg, R. Social Inequalities in Physical Activity Implications of Research Practices–The Case of Studying Breast Cancer Survivors [Doctoral Thesis]. Trondheim: Norwegian university of science and technology (2021).
66. Akobeng, AK. Assessing the Validity of Clinical Trials. J Pediatr Gastroenterol Nutr (2008) 47(3):277–82. doi:10.1097/MPG.0b013e31816c749f
67. Campbell, DT. Factors Relevant to the Validity of Experiments in Social Settings. Psychol Bull (1957) 54(4):297–312. doi:10.1037/h0040950
68. World Medical Association. World Medical Association Declaration of helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA (2013) 310(20):2191–4. doi:10.1001/jama.2013.281053
69. Rennie, DJJ. CONSORT Revised—Improving the Reporting of Randomized Trials. JAMA (2001) 285(15):2006–7.doi:10.1001/jama.285.15.2006
70. Schulz, KF, Altman, DG, and Moher, D, CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMC Med (2010) 8:18. doi:10.1186/1741-7015-8-18
71. Speck, RM, Courneya, KS, Masse, LC, Duval, S, and Schmitz, KH. An Update of Controlled Physical Activity Trials in Cancer Survivors: A Systematic Review and Meta-Analysis. J Cancer Survivorship (2010) 4(2):87–100. doi:10.1007/s11764-009-0110-5
72. Furler, J, Magin, P, Pirotta, M, and van Driel, M. Participant Demographics Reported in "Table 1" of Randomised Controlled Trials: A Case of "inverse Evidence. Int J Equity Health (2012) 11:14. doi:10.1186/1475-9276-11-14
73. Stalsberg, R, and Pedersen, AV. Effects of Socioeconomic Status on the Physical Activity in Adolescents: A Systematic Review of the Evidence. Scand J Med Sci Sports (2010) 20(3):368–83. doi:10.1111/j.1600-0838.2009.01047.x
74. Gidlow, C, Johnston, LH, Crone, D, Ellis, N, and James, D. A Systematic Review of the Relationship between Socio-Economic Position and Physical Activity. Health Edu J (2006) 65(4):338–67. doi:10.1177/0017896906069378
75. Evans, JA, and Foster, JG. Metaknowledge. Science (2011) 331(6018):721–5. doi:10.1126/science.1201765
76. Schatz, BR. Information Retrieval in Digital Libraries: Bringing Search to the Net. Science (1997) 275(5298):327–34. doi:10.1126/science.275.5298.327
77. Bramer, WM, De Jonge, GB, Rethlefsen, ML, Mast, F, and Kleijnen, J. A Systematic Approach to Searching: An Efficient and Complete Method to Develop Literature Searches. J Med Libr Assoc JMLA. (2018) 106(4):531–41. doi:10.5195/jmla.2018.283
78. Horsley, T, Dingwall, O, and Sampson, M. Checking Reference Lists to Find Additional Studies for Systematic Reviews. Cochrane Db Syst Rev (2011) 2011(8):MR000026. doi:10.1002/14651858.MR000026.pub2
79. Hu, X, Rousseau, R, and Chen, J. On the Definition of Forward and Backward Citation Generations. J Informetrics (2011) 5(1):27–36. doi:10.1016/j.joi.2010.07.004
80. Rysstad, AL, and Pedersen, AV. Brief Report: Non-right-Handedness within the Autism Spectrum Disorder. J autism Dev Disord (2016) 46(3):1110–7. doi:10.1007/s10803-015-2631-2
81. Darvik, M, Lorås, HW, and Pedersen, AV. The Prevalence of Left-Handedness Is Higher Among Individuals with Developmental Coordination Disorder Than in the General Population. Front Psychol (2018) 9:1948–11. doi:10.3389/fpsyg.2018.01948
82. The World Bank. Higher Education. THE WORLD BANK (2021). Available from: https://www.worldbank.org/en/topic/tertiaryeducation (Accessed December 1, 2023).
83. World Bank – processed by Our World in Data. Average Years of Schooling for Women [dataset]. World Bank, World Bank Education Statistics (EdStats) (2023). Available from: https://ourworldindata.org/grapher/mean-years-of-schooling-female?tab=table&time=2004.2005 (Accessed November 7, 2023).
84. World Bank – processed by Our World in Data. Average Years of Schooling for Women [dataset]. World Bank, World Bank Education Statistics (EdStats) (2023). Available from: https://ourworldindata.org/grapher/mean-years-of-schooling-female?tab=table&time=2006.2009 (Accessed November 7, 2023).
85. OECD. Educational Attainment and Labour-Force Status: Trends in Educational Attainment, by Educational Attainment and Age Group: OECD.Stat (2023). Available from: https://stats.oecd.org/Index.aspx?QueryId=93191 (Accessed November 7, 2023).
86. Ministry of Education, Republic of China (Taiwan). Enrollment Rates of School - Total Net Enrollment Rates: Ministry of Education (2022). Available from: https://stats.moe.gov.tw/files/ebook/indicators/13.pdf (Accessed May 04, 2022).
87. United Nations. Department of Economic and Social Affairs Population Division: Estimates and Projections of Women of Reproductive Age Who Are Married or in a Union (2022). Available from: https://population.un.org/dataportal/data/indicators/44/locations/840,124,156,158,250,528,826,724,208,752,76/start/2000/end/2020/table/pivotbyvariant (Accessed November 7, 2023).
88. Statistics Canada. Canada (Code 01) (Table). National Household Survey (NHS) Profile. 2011 National Household Survey. Statistics Canada Catalogue No. 99-004-XWE (2013). Available from: https://www12.statcan.gc.ca/nhs-enm/2011/dp-pd/prof/details/page.cfm?Lang=E&Geo1=PR&Code1=01&Data=Count&SearchText=Canada&SearchType=Begins&SearchPR=01&A1=All&B1=All&Custom=&TABID=1 (Accessed September 11, 2013).
89. Statistics Canada. Canada [Country] and Canada [Country] (Table). Census Profile. 2016 Census. Statistics Canada Catalogue No. 98-316-X2016001 (2017). Available from: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=PR&Code1=01&Geo2=PR&Code2=01&Data=Count&SearchText=canada&SearchType=Begins&SearchPR=01&B1=All&TABID=1 (Accessed November 29, 2017).
90. Statistics Canada. Visible Minority and Population Group by Generation Status: Canada, Provinces and Territories, Census Metropolitan Areas and Census Agglomerations with Parts. Table: 98-10-0324-01 (2023). Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=9810032401 (Accessed October 26, 2023).
91. United States Census Bureau. Race and Ethnicity in the United States: 2010 Census and 2020 Census 2021 (2021). Available from: https://www.census.gov/library/visualizations/interactive/race-and-ethnicity-in-the-united-state-2010-and-2020-census.html (Accessed November 29, 2023).
92. United States Census Bureau. "Profile of General Demographic Characteristics: 2000." Decennial Census, DEC State Legislative District Demographic Profile (100-Percent) (2000). Available from: https://data.census.gov/table?y=2000 (Accessed November 29, 2023).
93. Pickett, M, Mock, V, Ropka, ME, Cameron, L, Coleman, M, and Podewils, L. Adherence to Moderate-Intensity Exercise during Breast Cancer Therapy. Cancer Pract (2002) 10(6):284–92. doi:10.1046/j.1523-5394.2002.106006.x
94. Al-Majid, S, Wilson, LD, Rakovski, C, and Coburn, JW. Effects of Exercise on Biobehavioral Outcomes of Fatigue during Cancer Treatment: Results of a Feasibility Study. Biol Res Nurs (2015) 17(1):40–8. doi:10.1177/1099800414523489
95. Courneya, KS, McKenzie, DC, Mackey, JR, Gelmon, K, Friedenreich, CM, Yasui, Y, et al. Effects of Exercise Dose and Type during Breast Cancer Chemotherapy: Multicenter Randomized Trial. J Natl Cancer (2013) 105(23):1821–32. doi:10.1093/jnci/djt297
96. Anderson, RT, Kimmick, GG, McCoy, TP, Hopkins, J, Levine, E, Miller, G, et al. A Randomized Trial of Exercise on Well-Being and Function Following Breast Cancer Surgery: The RESTORE Trial. J Cancer Survivorship-Research Pract (2012) 6(2):172–81. doi:10.1007/s11764-011-0208-4
97. Arem, H, Sorkin, M, Cartmel, B, Fiellin, M, Capozza, S, Harrigan, M, et al. Exercise Adherence in a Randomized Trial of Exercise on Aromatase Inhibitor Arthralgias in Breast Cancer Survivors: The Hormones and Physical Exercise (HOPE) Study. J Cancer Survivorship (2016) 10(4):654–62. doi:10.1007/s11764-015-0511-6
98. Ariza-Garcia, A, Lozano-Lozano, M, Galiano-Castillo, N, Postigo-Martin, P, Arroyo-Morales, M, and Cantarero-Villanueva, I. A Web-Based Exercise System (E-CuidateChemo) to Counter the Side Effects of Chemotherapy in Patients with Breast Cancer: Randomized Controlled Trial. J Med Internet Res (2019) 21(7):e14418. doi:10.2196/14418
99. Bland, KA, Kirkham, AA, Bovard, J, Shenkier, T, Zucker, D, McKenzie, DC, et al. Effect of Exercise on Taxane Chemotherapy–Induced Peripheral Neuropathy in Women with Breast Cancer: A Randomized Controlled Trial. Clin Breast Cancer (2019) 19(6):411–22. doi:10.1016/j.clbc.2019.05.013
100. Cadmus, LA, Salovey, P, Yu, H, Chung, G, Kasl, S, and Irwin, ML. Exercise and Quality of Life during and after Treatment for Breast Cancer: Results of Two Randomized Controlled Trials. Psycho-Oncology (2009) 18(4):343–52. doi:10.1002/pon.1525
101. Carayol, M, Ninot, G, Senesse, P, Bleuse, J-P, Gourgou, S, Sancho-Garnier, H, et al. Short and Long-Term Impact of Adapted Physical Activity and Diet Counseling during Adjuvant Breast Cancer Therapy: The “APAD1” Randomized Controlled Trial. BMC cancer (2019) 19(1):737–20. doi:10.1186/s12885-019-5896-6
102. Chandwani, KD, Thornton, B, Perkins, GH, Arun, B, Raghuram, N, Nagendra, H, et al. Yoga Improves Quality of Life and Benefit Finding in Women Undergoing Radiotherapy for Breast Cancer. J Soc Integr Oncol (2010) 8(2):43–55.
103. Chen, Z, Meng, Z, Milbury, K, Bei, W, Zhang, Y, Thornton, B, et al. Qigong Improves Quality of Life in Women Undergoing Radiotherapy for Breast Cancer: Results of a Randomized Controlled Trial. Cancer (2013) 119(9):1690–8. doi:10.1002/cncr.27904
104. Courneya, KS, Segal, RJ, Gelmon, K, Reid, RD, Mackey, JR, Friedenreich, CM, et al. Predictors of Supervised Exercise Adherence during Breast Cancer Chemotherapy. Med Sci Sports Exerc (2008) 40:1180–7. doi:10.1249/MSS.0b013e318168da45
105. Demark-Wahnefried, W, Case, LD, Blackwell, K, Marcom, PK, Kraus, W, Aziz, N, et al. Results of a Diet/exercise Feasibility Trial to Prevent Adverse Body Composition Change in Breast Cancer Patients on Adjuvant Chemotherapy. Clin Breast Cancer (2008) 8(1):70–9. doi:10.3816/CBC.2008.n.005
106. Gokal, K, Wallis, D, Ahmed, S, Boiangiu, I, Kancherla, K, and Munir, F. Effects of a Self-Managed home-Based Walking Intervention on Psychosocial Health Outcomes for Breast Cancer Patients Receiving Chemotherapy: A Randomised Controlled Trial. Support Care Cancer (2016) 24(3):1139–66. doi:10.1007/s00520-015-2884-5
107. Huang, HP, Wen, FH, Tsai, JC, Lin, YC, Shun, SC, Chang, HK, et al. Adherence to Prescribed Exercise Time and Intensity Declines as the Exercise Program Proceeds: Findings from Women under Treatment for Breast Cancer. Support Care Cancer (2015) 23(7):2061–71. doi:10.1007/s00520-014-2567-7
108. Kirkham, AA, Van Patten, CL, Gelmon, KA, McKenzie, DC, Bonsignore, A, Bland, KA, et al. Effectiveness of Oncologist-Referred Exercise and Healthy Eating Programming as a Part of Supportive Adjuvant Care for Early Breast Cancer. Oncologist (2018) 23(1):105–15. doi:10.1634/theoncologist.2017-0141
109. Lund, LW, Ammitzbøll, G, Hansen, DG, Andersen, EAW, and Dalton, SO. Adherence to a Long-Term Progressive Resistance Training Program, Combining Supervised and home-Based Exercise for Breast Cancer Patients during Adjuvant Treatment. Acta Oncologica (2019) 58(5):650–7. doi:10.1080/0284186X.2018.1560497
110. Matthews, CE, Wilcox, S, Hanby, CL, Ananian, CD, Heiney, SP, Gebretsadik, T, et al. Evaluation of a 12-Week home-Based Walking Intervention for Breast Cancer Survivors. Support Care Cancer (2007) 15(2):203–11. doi:10.1007/s00520-006-0122-x
111. Mijwel, S, Backman, M, Bolam, KA, Jervaeus, A, Sundberg, CJ, Margolin, S, et al. Adding High-Intensity Interval Training to Conventional Training Modalities: Optimizing Health-Related Outcomes during Chemotherapy for Breast Cancer: The OptiTrain Randomized Controlled Trial. Breast Cancer Res Tr (2018) 168(1):79–93. doi:10.1007/s10549-017-4571-3
112. Mock, V, Frangakis, C, Davidson, NE, Ropka, ME, Pickett, M, Poniatowski, B, et al. Exercise Manages Fatigue during Breast Cancer Treatment: A Randomized Controlled Trial. Psycho-Oncology (2005) 14(6):464–77. doi:10.1002/pon.863
113. Paulo, TR, Rossi, FE, Viezel, J, Tosello, GT, Seidinger, SC, Simões, RR, et al. The Impact of an Exercise Program on Quality of Life in Older Breast Cancer Survivors Undergoing Aromatase Inhibitor Therapy: A Randomized Controlled Trial. Health Qual Life Out (2019) 17(1):17–2. doi:10.1186/s12955-019-1090-4
114. Ratcliff, CG, Milbury, K, Chandwani, KD, Chaoul, A, Perkins, G, Nagarathna, R, et al. Examining Mediators and Moderators of Yoga for Women with Breast Cancer Undergoing Radiotherapy. Integr Cancer Therapies (2016) 15(3):250–62. doi:10.1177/1534735415624141
115. D Reis, ME Walsh, S Young-McCaughan, and T Jones, Effects of Nia Exercise in Women Receiving Radiation Therapy for Breast Cancer . Oncol Nurs Forum (2013).
116. Rogers, LQ, Hopkins-Price, P, Vicari, S, Pamenter, R, Courneya, KS, Markwell, S, et al. A Randomized Trial to Increase Physical Activity in Breast Cancer Survivors. Med Sci Sports Exerc (2009) 41(4):935–46. doi:10.1249/MSS.0b013e31818e0e1b
117. Travier, N, Velthuis, MJ, Bisschop, CNS, van den Buijs, B, Monninkhof, EM, Backx, F, et al. Effects of an 18-Week Exercise Programme Started Early during Breast Cancer Treatment: A Randomised Controlled Trial. Bmc Med (2015) 13:121. doi:10.1186/s12916-015-0362-z
118. Vadiraja, SH, Rao, MR, Nagendra, RH, Nagarathna, R, Rekha, M, Vanitha, N, et al. Effects of Yoga on Symptom Management in Breast Cancer Patients: A Randomized Controlled Trial. Int J Yoga (2009) 2(2):73–9. doi:10.4103/0973-6131.60048
119. Vallance, JK, Friedenreich, CM, Lavallee, CM, Culos-Reed, N, Mackey, JR, Walley, B, et al. Exploring the Feasibility of a Broad-Reach Physical Activity Behavior Change Intervention for Women Receiving Chemotherapy for Breast Cancer: A Randomized Trial. Cancer Epidemiol Biomarkers Prev (2016) 25(2):391–8. doi:10.1158/1055-9965.EPI-15-0812
120. van Waart, H, Stuiver, MM, van Harten, WH, Geleijn, E, Kieffer, JM, Buffart, LM, et al. Effect of Low-Intensity Physical Activity and Moderate to High-Intensity Physical Exercise during Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol (2015) 33(17):1918–27. doi:10.1200/JCO.2014.59.1081
121. Wang, YJ, Boehmke, M, Wu, YW, Dickerson, SS, and Fisher, N. Effects of a 6-Week Walking Program on Taiwanese Women Newly Diagnosed with Early-Stage Breast Cancer. Cancer Nurs (2011) 34(2):E1–13. doi:10.1097/NCC.0b013e3181e4588d
122. Zhou, K, Wang, W, An, J, Li, M, Li, J, and Li, X. Effects of Progressive Upper Limb Exercises and Muscle Relaxation Training on Upper Limb Function and Health-Related Quality of Life Following Surgery in Women with Breast Cancer: A Clinical Randomized Controlled Trial. Ann Surg Oncol (2019) 26(7):2156–65. doi:10.1245/s10434-019-07305-y
123. DeNysschen, CA, Brown, JK, Cho, MH, and Dodd, MJ. Nutritional Symptom and Body Composition Outcomes of Aerobic Exercise in Women with Breast Cancer. Clin Nurs Res (2011) 20(1):29–46. doi:10.1177/1054773810379402
124. Dieli-Conwright, CM, Parmentier, J-H, Sami, N, Lee, K, Spicer, D, Mack, WJ, et al. Adipose Tissue Inflammation in Breast Cancer Survivors: Effects of a 16-Week Combined Aerobic and Resistance Exercise Training Intervention. Breast Cancer Res Tr (2018) 168(1):147–57. doi:10.1007/s10549-017-4576-y
125. Kim, C-J, Kang, D-H, Smith, BA, and Landers, KA. Cardiopulmonary Responses and Adherence to Exercise in Women Newly Diagnosed with Breast Cancer Undergoing Adjuvant Therapy. Cancer Nurs (2006) 29(2):156–65. doi:10.1097/00002820-200603000-00013
126. Lee, K, Kang, I, Mack, WJ, Mortimer, J, Sattler, F, Salem, G, et al. Feasibility of High Intensity Interval Training in Patients with Breast Cancer Undergoing Anthracycline Chemotherapy: A Randomized Pilot Trial. BMC cancer (2019) 19(1):653–9. doi:10.1186/s12885-019-5887-7
127. Stan, DL, Kathleen Sundt, R, Cheville, AL, Youdas, JW, Krause, DA, Boughey, JC, et al. Pilates for Breast Cancer Survivors: Impact on Physical Parameters and Quality of Life after Mastectomy. Clin J Oncol Nurs (2012) 16(2):131–41. doi:10.1188/12.CJON.131-141
128. Swenson, KK, Nissen, MJ, Anderson, E, Shapiro, A, Schousboe, J, and Leach, J. Effects of Exercise vs Bisphosphonates on Bone mineral Density in Breast Cancer Patients Receiving Chemotherapy. J Support Oncol (2009) 7(3):101–7.
129. Yang, CY, Tsai, JC, Huang, YC, and Lin, CC. Effects of a home-Based Walking Program on Perceived Symptom and Mood Status in Postoperative Breast Cancer Women Receiving Adjuvant Chemotherapy. J Adv Nurs (2011) 67(1):158–68. doi:10.1111/j.1365-2648.2010.05492.x
130. Ainsworth, BE, Haskell, WL, Herrmann, SD, Meckes, N, Bassett, DR, Tudor-Locke, C, et al. 2011 Compendium of Physical Activities: A Second Update of Codes and MET Values. Med Sci Sports Exerc (2011) 43(8):1575–81. doi:10.1249/MSS.0b013e31821ece12
131. van Waart, H, van Harten, WH, Buffart, LM, Sonke, GS, Stuiver, MM, and Aaronson, NK. Why Do Patients Choose (Not) to Participate in an Exercise Trial during Adjuvant Chemotherapy for Breast Cancer? Psycho-Oncology (2016) 25(8):964–70. doi:10.1002/pon.3936
132. Susukida, R, Crum, RM, Stuart, EA, Ebnesajjad, C, and Mojtabai, R. Assessing Sample Representativeness in Randomized Controlled Trials: Application to the National Institute of Drug Abuse Clinical Trials Network. Addiction (2016) 111(7):1226–34. doi:10.1111/add.13327
133. Galobardes, B, Shaw, M, Lawlor, DA, Lynch, JW, and Smith, GD. Indicators of Socioeconomic Position (Part 1). J Epidemiol Community Health (2006) 60(1):7–12. doi:10.1136/jech.2004.023531
134. Murthy, VH, Krumholz, HM, and Gross, CP. Participation in Cancer Clinical Trials: Race, Sex, and Age-Based Disparities. Jama (2004) 291(22):2720–6. doi:10.1001/jama.291.22.2720
135. Semega, J, and Kollar, M. Income in the United States: 2021. United States: U.S. CENSUS BUREAU (2022).
136. Sims, JM. A Brief Review of the Belmont Report. Dimensions Crit Care Nurs (2010) 29(4):173–4. doi:10.1097/DCC.0b013e3181de9ec5
137. Coughlin, SS. Social Determinants of Breast Cancer Risk, Stage, and Survival. Breast Cancer Res Treat (2019) 177:537–48. doi:10.1007/s10549-019-05340-7
138. Stalsberg, R, Bertheussen, GF, Børset, H, Thomsen, SN, Husøy, A, Flote, VG, et al. Do Breast Cancer Patients Manage to Participate in an Outdoor, Tailored, Physical Activity Program during Adjuvant Breast Cancer Treatment, Independent of Health and Socio-Demographic Characteristics? J Clin Med (2022) 11(3):843. doi:10.3390/jcm11030843
139. Daley, AJ, Crank, H, Mutrie, N, Saxton, JM, and Coleman, R. Determinants of Adherence to Exercise in Women Treated for Breast Cancer. Eur J Oncol Nurs (2007) 11(5):392–9. doi:10.1016/j.ejon.2007.03.001
140. Brunet, J, Taran, S, Burke, S, and Sabiston, CM. A Qualitative Exploration of Barriers and Motivators to Physical Activity Participation in Women Treated for Breast Cancer. Disabil Rehabil (2013) 35(24):2038–45. doi:10.3109/09638288.2013.802378
141. Strazdins, L, Griffin, AL, Broom, DH, Banwell, C, Korda, R, Dixon, J, et al. Time Scarcity: Another Health Inequality? Environ Plann A: Economy Space (2011) 43(3):545–59. doi:10.1068/a4360
142. Strazdins, L, Welsh, J, Korda, R, Broom, D, and Paolucci, F. Not All Hours Are Equal: Could Time Be a Social Determinant of Health? Sociol Health Ill (2016) 38(1):21–42. doi:10.1111/1467-9566.12300
143. Phelan, JC, Link, BG, Diez-Roux, A, Kawachi, I, and Levin, B. “Fundamental Causes” of Social Inequalities in Mortality: A Test of the Theory. J Health Soc Behav (2004) 45(3):265–85. doi:10.1177/002214650404500303
Keywords: sampling bias, social validity, research equity, exercise trials, breast cancer, mammae cancer, meta-analysis
Citation: Stalsberg R and Darvik MD (2024) Social Representativeness and Intervention Adherence—A Systematic Review of Clinical Physical Activity Trials in Breast Cancer Patients. Int J Public Health 69:1607002. doi: 10.3389/ijph.2024.1607002
Received: 21 December 2023; Accepted: 10 April 2024;
Published: 09 May 2024.
Edited by:
Cyrille Delpierre, INSERM Public Health, FranceReviewed by:
Christian Janssen, Munich University of Applied Sciences, GermanyOne reviewer who chose to remain anonymous
Copyright © 2024 Stalsberg and Darvik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ragna Stalsberg, cmFnbmEuc3RhbHNiZXJnQG50bnUubm8=
 Ragna Stalsberg
Ragna Stalsberg Monica Dahle Darvik
Monica Dahle Darvik