- Department of Public Health, Arba Minch College of Health Sciences, Arba Minch, Ethiopia
Objectives: Despite increased access to and availability of antiretroviral therapy, the program’s effectiveness is primarily affected by treatment adherence. Therefore, this study aimed to determine the magnitude and predictors of suboptimal adherence among children on ART in Southern Ethiopia.
Methods: A multicenter retrospective study was conducted among human immunodeficiency virus (HIV) infected children in Gamo and South Omo zone public health facilities. To identify factors associated with suboptimal adherence, a binary logistic regression model was fitted. Variables with a p-value ≤0.25 in bivariable logistic regression analysis were included in multivariable logistic regression analysis. p-value <0.05 was used to declare statistical significance.
Results: The suboptimal adherence was determined to be 30.3% (95% CI: 25.5%, 35.6%). Advanced clinical stage, hemoglobin level <10 mg/dL, unchanged initial regimen, and non-disclosure of HIV sero-status were significant predictors of suboptimal adherence.
Conclusion: Suboptimal adherence is a significant public health problem in the study setting. Therefore, designing interventions towards improving adherence is needed especially for children with poor clinical characteristics.
Introduction
Human immunodeficiency virus (HIV) remains a major global public health problem, having claimed 40.1 million lives so far with ongoing transmission in all countries globally. It is an infection that attacks the body’s immune system, and acquired immunodeficiency syndrome (AIDS) is the most advanced stage of the disease [1]. By the year 2020, about 1.7 million children under 15 years old were living with Human Immunodeficiency Virus (HIV) [2], and about 99,000 died of AIDS-related mortality worldwide [3]. Sub-Saharan Africa is the region most affected by HIV and about 90% of all adolescents and children living with HIV were living in the region [4]. Ethiopia is one of the Sub-Saharan African countries with a total of 43,400 children living with HIV, out of which 2,000 AIDS-related mortalities occurred by the year 2020 [5].
Adherence is described as “the extent to which a person’s behavior, such as taking medication, sticking to a diet, and/or implementing lifestyle changes, corresponds with agreed-upon recommendations from a healthcare provider” [6]. Any child who misses more than three dosages throughout a 1 month treatment term is considered to have less than 95% adherence, which is suboptimal adherence, and a child who misses a dose of fewer than three is considered to have optimal adherence [7]. The success and duration of ART drug regimens are mostly influenced by treatment adherence, which needs near-perfect adherence of up to 95% [8–10]. Suboptimal adherence among children remains a significant challenge for ART programs in both developed and developing countries [11]. According to evidence from studies conducted in numerous countries throughout the world, the extent of suboptimal adherence among HIV-positive children was expected to be substantial. In a systematic review conducted in low- and middle-income countries among children living with HIV, the level of adherence ranges from 49% to 100% [12]. Studies conducted in Sub-Saharan African countries showed that the adherence level was reported to be from 29% to 98% among children on ART [13–15]. Evidence from previous studies conducted in different parts of Ethiopia revealed that the magnitude of suboptimal adherence to ART ranged from 9.3% in Hossana [16] to 34% in Southwest Ethiopia [17].
Suboptimal treatment adherence is linked with an increase in the risk of unfavorable treatment outcomes like treatment failure and antiretroviral drug resistance, immunological decline resulting in opportunistic infections, and advanced HIV disease progression [18–22]. This in turn leads to preventable morbidity and mortality, increased expenditure for care, and avoidable forward transmission of HIV [23]. Poor adherence to ART reduces the effectiveness of viral suppression, increases viral resistance, and puts people living with HIV (PLHIV) at risk of hospital admission, and opportunistic infection [24]. Moreover, the effect of suboptimal adherence was also revealed by the outcome of the ambitious 90–90–90 targets, which aimed to attain 90% viral suppression. Due to this effect, only 40% of under-fifteen-year-old children living with HIV were virally suppressed [25].
Evidence from previous studies identified different factors associated with suboptimal treatment adherence of HIV-positive children on ART. These include the child’s age, the caregiver’s age, TB co-infection, advanced clinical stage, non-disclosure of children’s sero-status, and treatment failure [14, 26, 27].
Several strategies have been implemented to improve treatment adherence in children on ART, and they have shown benefits in improving adherence. Mobile phone text messages, behavioral skills training/medication adherence training for caregivers, fixed-dose combinations, and once-daily regimens are among the approaches [28]. Despite these efforts, poor adherence remains a significant hurdle to the efficacy of ART programs. The degree of suboptimal adherence level and factors associated with it can vary from place to place, so it is necessary to assess the problem in order to design effective strategies. However, there is limited evidence regarding suboptimal adherence to care among children on ART in Ethiopia, and no evidence in a study setting. Therefore, this study aimed to assess suboptimal adherence and associated factors among children receiving ART.
Methods
Study Design, Period, and Setting
An institution-based retrospective follow-up study was conducted from 12 April to 10 May 2022. The study was conducted in public health facilities found in Gamo, and South Omo zones, Southern Ethiopia. Arba Minch town is 505 Kilometers (KM) Southwest far from the capital city, Addis Ababa. Jinka town is about 563 KM far from Addis Ababa and 399 Kilometers from Hawassa. In these two zones, there are about 23 health facilities (2 general hospitals, 5 primary hospitals, and 16 health centers) that are currently providing pediatric ART services. These health facilities provide different services to the community in their catchment area and nearby woredas and zones. In these two zones currently, there are about 256 HIV-positive children on active ART follow-up. The follow-up schedule was based on the national ART guideline of Ethiopia; children beginning their follow-up returned for their first follow-up after 2 weeks of initiation of ART then monthly for the first 6 months, followed by every 3 months for drug refill, clinical assessment, and adherence support [23].
Population
All HIV-positive children (<15 years) who were on ART in public health facilities of Gamo and South Omo Zones made up the source population. All HIV-positive children (<15 years) who were on ART in Jinka General Hospital, Arba Minch General Hospital, Gazer Primary Hospital, Chencha Primary Hospital, Birbir Health Center, Gerese Health Center, Jinka Millennium Health Center, Kamba Health Center, and Shele Health Center, from 1 January 2012 to 31 December 2021 made up the study population. All HIV-positive children (<15 years) who were on ART and had completed at least one follow-up visit were included in the study. Children on ART with incomplete records were excluded from the study.
Sample Size Determination and Sampling Technique
Sample Size Determination
The sample size was determined by using a single population proportion formula [n = [(Za/2)2*P (1−P)]/d2] and by considering a 95% confidence interval with a confidence level of Za/2 = 1.96, the proportion of sub-optimal adherence of 21.8% taken from a study conducted in south Gondar public hospitals, Northwest Ethiopia [26], a margin of error (d) 5%. Using the above assumptions, the calculated sample size was 262 and after adding a 10% incompleteness rate, the final sample size was 289.
Sampling Technique and Procedure
The public health facilities in Gamo and South Omo Zones were stratified based on their type of health facility into General Hospitals, Primary Hospitals, and Health Centers. Then by simple random sampling (lottery method) two Primary Hospitals and five Health Centers were selected. Both of the General Hospitals were included. The children <15 years of age were identified in each of the health facilities using medical record numbers (MRN) that were obtained from electronic databases. Patients’ charts were drawn using the MRN. In these health facilities 349 children (age < 15) on ART from 01 January 2012 to 31 December 2021 were identified and 323 of the children who fulfilled the eligibility criteria within the follow-up period were included in the study.
Data Collection Tools and Procedures
Data were collected by using a data extraction checklist developed in English from the standardized ART intake and follow-up forms from national HIV guideline [23], and by reviewing related literatures. The tool contains socio-demographic, clinical, and treatment-related characteristics of participants. The lists of study participants were taken from the ART data clerk by using children’s MRN or unique ART numbers. Charts of the children were taken from card rooms. Then data were collected by reviewing the patient follow-up charts by fourteen data collectors and three supervisors.
Operational Definitions
Suboptimal adherence: If HIV-positive children on ART experienced fair or poor adherence (drug adherence of ≤94% or ≥3 doses missed monthly) [23].
CD4 count for severe immunodeficiency: The classification was based on children’s age. For children < 5 years CD4 < 200 cells/mm3, and CD4 < 100 cells/mm3 children ≥ 5 years [29].
Nutritional status: Was measured by weight for age (WFA) and body mass index (BMI) for Age. Categorized as; underweight (WFA or BMI for age <−z-score) and normal (z-score > −2) [23].
Data Quality Assurance
The data collection process was conducted by 14 data collectors (9 BSc in Nursing and 5 BSc in Public Health), who were trained on comprehensive HIV care and providing follow-up care services, and three supervisors (BSc in Public Health) after receiving 1 day of training. To check the relevance, consistency, and adequacy of the checklist, a pretest was conducted before the actual data collection in the same setting. The data collection tool was properly numbered and coded. The principal investigator and supervisors carried out daily monitoring of the data collection process by reviewing and checking the filled-out checklists to ensure accuracy, completeness, and consistency.
Data Processing and Statistical Analysis
The collected data were entered into Epi-Data version 3.1 and then exported to STATA version 14.0 for management and analysis. Exploratory data analysis was done to check the presence of potential outliers, normality, and level of missing values. Z-scores, to assess nutritional status, were generated by using WHO Anthro-Plus software. Descriptive statistics were found using mean, median, standard deviation, interquartile range, frequencies, and percentages. A bivariable logistic regression model was fitted to assess the association between each independent variable and the dependent variable, variables with a p-value ≤ 0.25 in bivariable logistic regression were candidates for multivariable analysis. A multivariable logistic regression model with a backward likelihood ratio method was fitted to identify factors significantly associated with suboptimal adherence. Multicollinearity was checked by using variance inflation factor (VIF) and tolerance, the mean VIF = 1.08, indicating no threat of collinearity. The goodness of fit of the model was checked by the Hosmer-Lemeshow chi-square test (Prob > X2 = 0.2309). AOR with a 95% CI and corresponding p-value was used to identify statistically significant variables. p-value <0.05 was used to declare statistical significance.
Result
From a total of 349 children (age <15 years) who were receiving ART from 1st January 2012 to 31st December 2021, about 323 have fulfilled the inclusion criteria and are included in the analysis with a 92.6% completeness rate of charts.
Sociodemographic Characteristics of Children Receiving ART
The median age of the study participants was 5 (IQR: 2–9) years, 118 (36.5%) children were in the age group of ≤3 years, and approximately a quarter (91 (28.2%)) of the participants were in the age group of ≥9 years. Among the study participants, 167 (51.7%) were male and forty-five children (13.9%) had lost both of their parents. For the majority of the children, 277 (85.8%) caregivers were their biological families. Three percent of the caregivers were in the age range of ≤24 years and 135 (41.8%) of the caregivers were in the age group of 35–44 years. Regarding the place of residence, more than half (50.8%) of the HIV-positive children were urban residents. Half of the caregivers [165 (51.1%)] had not attended formal education and 28 (8.6%) attained a tertiary and above educational level. One-third of the caregivers [112 (34.6%)] were daily laborers and 42 (13%) were government employees (Table 1).
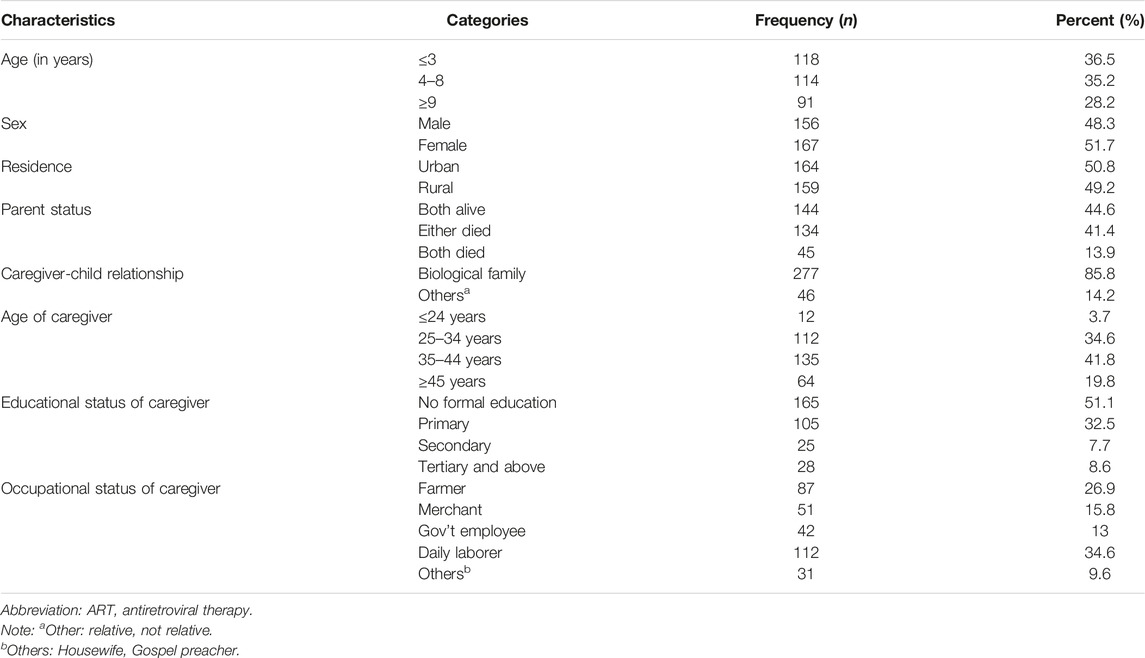
TABLE 1. Socio-demographic characteristics of children on antiretroviral therapy in public health facilities of Gamo and South Omo zones, southern Ethiopia, 2022 (n = 323) (Predictors of Suboptimal Adherence Among Children on Antiretroviral Therapy in Southern Ethiopia, 2022: A Multicenter Retrospective Follow-Up Study).
Clinical and Treatment-Related Characteristics of Children on ART
Among the 323 HIV-positive children who participated in the study, more than three-quarters (78.0%) were from hospitals, and the remaining 71 (22.0%) were from health centers. One hundred sixty-four (50.8%) HIV-positive children were enrolled with a test-and-treat approach. Regarding the BMI for age z-score, 222 (68.7%) of the study participants had a BMI for age z-score of ≥−2. Regarding baseline weight for age and height for age z-scores, 33.6% and 32.5% of children had a z-score of <−2, respectively. Among the under-five children who participated in the study, 99 (65.1%) had reached a developmental milestone that was appropriate for their age, whereas 53 (34.8%) had a delayed or regressed developmental milestone. The functional status of the HIV-positive children aged above 5 years was identified to be 49.1%, 45.6%, and 5.3%, labeled as working, ambulatory, and bedridden, respectively. More than eighty percent of the participants had a hemoglobin level of ≥10 mg/dL and the mean hemoglobin level was 12.5 mg/dL (2.45 mg/dL SD). Concerning the WHO clinical stage, of the total children who participated in the study, 209 (64.7%) were in clinical stage I or II. The study reported that 45 (13.9%) HIV-positive children had a history of TB/HIV co-infection. In addition, 68 (21.1%) of the participants had a history of opportunistic infections other than TB. For the prevention of common opportunistic infections, 157 (48.6%) of the children on ART took both CPT and IPT. The median baseline CD4 count was determined to be 473 (IQR: 288–783) cells/mm3. Regarding ART regimens initiated during enrollment, 172 (53.3%) were started on Zidovudine, Lamivudine, and Nevirapine (AZT+3TC+NVP)-containing regimens, and only 21 (6.5%) were initiated on Dolutegravir (DTG)-containing regimens. The HIV sero-status of 72 (22.3%) children on ART was disclosed to the children themselves. The initial ART regimen of 149 (46.1%) children was not changed, and the children were taking their old ART regimen. Availability of new drugs was the main reason for regimen change, but other reasons, such as treatment failure, drug stockouts, and other unknown reasons, were also responsible. Only four children on ART experienced side effects.
Predictors of Suboptimal Treatment Adherence
This study identified that the magnitude of suboptimal adherence was 30.3% (95% CI: 25.5%, 35.6%) (Figure 1).
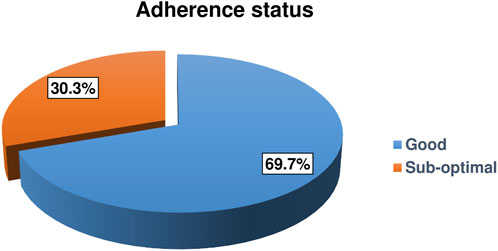
FIGURE 1. Adherence status of HIV-positive children on antiretroviral therapy in Gamo and South Omo zones public health facilities, Southern Ethiopia, 2022 (n = 323) (Predictors of Suboptimal Adherence Among Children on Antiretroviral Therapy in Southern Ethiopia, 2022: A Multicenter Retrospective Follow-Up Study).
In bivariable logistic regression analysis, suboptimal adherence was significantly associated with; sex of the child, parent status, educational status of caregiver, type of health facility, BMI for age, WHO clinical stage, history of tuberculosis infection, CPT prophylaxis, IPT prophylaxis, hemoglobin level, CD4 count, regiment change, and disclosure status at p-value of <0.25. In multivariable logistic regression analysis, WHO clinical stage, hemoglobin level, regimen change, and disclosure status of the children showed statistically significant association with suboptimal adherence at p-value <0.05.
The odds of suboptimal adherence was doubled (AOR = 2.29; 95% CI: 1.13, 3.98) among children with a WHO clinical stage of III or IV when compared to those with a WHO clinical stage of I or II. Children receiving ART with hemoglobin levels below 10 mg/dL had 3.62 times increased odds of suboptimal adherence when compared to their counterparts (AOR = 3.62; 95% CI: 1.82, 7.19). The odds of suboptimal adherence was nearly three times higher among children whose initial regimen was not changed when compared to those children whose initial regimen was changed (AOR = 2.69; 95% CI: 1.55, 4.67). Children whose HIV sero-status was disclosed had 2.46 times increased odds of suboptimal adherence when compared to their counterparts (AOR = 2.46; 95% CI: 1.17, 5.19) (Table 2).
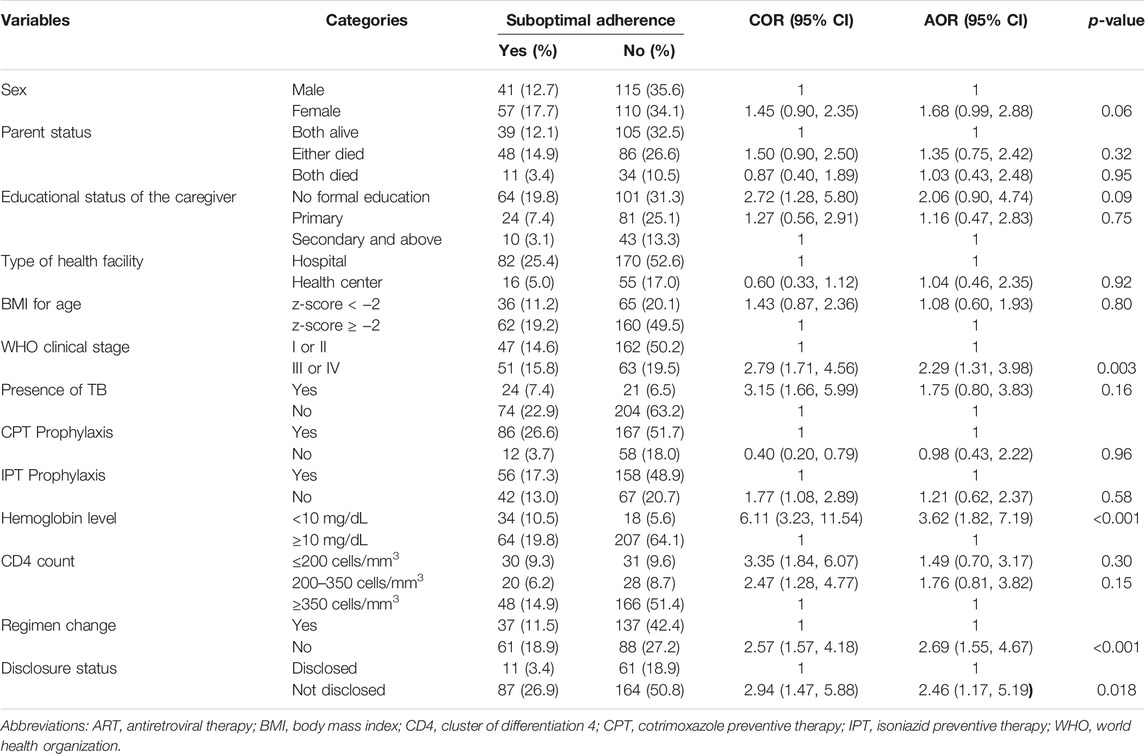
TABLE 2. Bivariable and multivariable analysis for predictors of suboptimal adherence among children on antiretroviral therapy in public health facilities of Gamo and South Omo zones, Southern Ethiopia, 2022 (n = 323) (Predictors of Suboptimal Adherence Among Children on Antiretroviral Therapy in Southern Ethiopia, 2022: A Multicenter Retrospective Follow-Up Study).
Discussion
This retrospective follow-up study was conducted to identify the predictors of suboptimal adherence among children on ART. As a result, advanced clinical stage (III or IV), low hemoglobin level, unchanged regime, and non-disclosure of HIV sero-status of the children were identified to be the independent predictors of suboptimal adherence.
This study revealed that the magnitude of suboptimal adherence was found to be 30.3%. This finding is lower than studies conducted in Dar es Salaam [14] and Northern Tanzania [30] which reported a magnitude of 40% and 75.4%, respectively. The result of this study is in line with the studies conducted in New Delhi [31] (34.4%) Uganda [32] (28.05%) Gondar in Ethiopia [33] (31.9%) and Ambo in Ethiopia [34] (33.3%). On the contrary, the magnitude of suboptimal adherence determined by this study was reported to be higher than previous studies conducted in South India [35] (9.1%) and Nigeria [36] (14%). Likewise, it was higher than studies previously conducted in different parts of Ethiopia which reported a magnitude of suboptimal adherence ranging from 5.16% to 21.8% among HIV-positive children who are on ART [26, 37–40].
The disparity could be attributed to differences in the adherence level diagnosis technique, the socio-demographic and cultural backgrounds of study participants, and patient-healthcare provider relationships in various settings. Socioeconomic status, study design, adherence assessment approaches, sample size, and setting differences may also be factors. Furthermore, under or over-reporting is more likely in low-income nations than in middle-income countries due to a shortage of expertise among healthcare personnel or caregivers. The consistency may be due to similarity in data recording formats and follow-up charts in the ART program for pediatric HIV Care and treatment in Ethiopia which was prepared by the Federal Minister of Health of Ethiopia [41].
In this study, the WHO clinical stage was associated with suboptimal treatment adherence among HIV-positive children on ART. Children with advanced clinical stage (stage III or IV) had twice the increased risk of suboptimal adherence when compared to those with a WHO clinical stage I or II. This is consistent with a study conducted in Dar es Salaam, Tanzania [14]. However, this result of the current studies is inconsistent with studies conducted in Ethiopia [34, 37, 42] which stated that children with advanced clinical stages (III/IV) were more likely to adhere. This may be due to the fact that children at advanced WHO clinical stages of the disease may be hopeless for their survival and hence may give little attention or even be ignorant of their medication as compared to their counterparts that are at stage I or II.
The current study reported that children receiving ART with hemoglobin levels below 10 mg/dL had 3.62 times increased odds of suboptimal adherence when compared to their counterparts. This is likely because of the exacerbation of previously existing anemia among children who started on a Zidovudine (AZT)-containing regimen, which can further lead to additional opportunistic infections and in turn reduce adherence due to the tension of the concurrent illness. In addition, it can also be because of the reduction in ART tolerance due to decreased absorption and the effect of immune defense from anemia.
Moreover, regimen change was another factor associated with suboptimal adherence, as identified by this study. The odds of suboptimal adherence was nearly three times higher among children whose initial regimen was not changed when compared to those children whose initial regimen was changed. This is consistent with a study done in Northwest Ethiopia [26] that states that children enrolled in the Zidovudine-containing ART regimen were more likely to be poor adherents. This regimen is the oldest one and is currently replaced with a Dolutegravir-containing regimen; hence, children on this old regimen have not had their regimen changed. This may be due to the fact that the majority of the old ART regimens have side effects that enhance the progression of the disease and result in subsequent complications. The Zidovudine-containing ART regimen was associated with anemia that imposed additional effects on the immune system.
Furthermore, the disclosure status of HIV-positive children on ART was found to be associated with suboptimal adherence, which is controversial. Therefore, children whose HIV sero-status was disclosed had 2.46 times higher odds of suboptimal adherence when compared to their counterparts. This is consistent with studies conducted in Uganda [32], Ethiopia [27, 33], and on the other hand, the report from the current study contradicts a study done in Tikur Anbessa Hospital, Ethiopia [42] that states that HIV-positive children who were not aware of their HIV sero-status were more likely to adhere. Children who are not aware of their HIV status may not grasp the rationale for taking drugs and may become resistant to them since they do not understand why they take medicine while appearing to be well. Children who are aware of their HIV sero-status may feel doomed, refuse, and intentionally miss the treatment doses until they accept their HIV sero-status, and the observed variation between studies may be associated with the extent and duration of counseling provided to children.
The study used a long year of data to identify predictors of suboptimal adherence among the children receiving ART. It is a retrospective study and used secondary data, the effect of some variables (like socioeconomic status and viral load) was not assessed because of incomplete recoding. Since the data were collected at a point in time retrospectively; it was difficult to ascertain potential cause-effect or temporal relationship.
Conclusion
The magnitude of suboptimal adherence was found to be high in the study settings. Advanced clinical stage, low hemoglobin level, unchanged regimen, and non-disclosure of HIV sero-status were the identified predictors of suboptimal adherence. To improve treatment adherence, special attention should be given to adherence counseling and training of adherence supports, especially for children with poor baseline clinical characteristics, those who are taking old regimens, and children with non-disclosed HIV sero-status. Researchers should conduct a longitudinal prospective study to address further clinical and Sociodemographic predictors of suboptimal adherence.
Ethics Statement
The Institutional Research Ethics Review Board (IRB) of Arba Minch University’s College of Medicine and Health Sciences granted ethical permission with the reference number IRB/123/2022. The study was carried out in line with the Helsinki Declaration on Health Research. Arba Minch University’s School of Public Health provided official support in the form of a letter. After explaining the purpose of the study, a letter of cooperation was submitted to administrators of public health facilities, and formal official permission was obtained for full access to the history and medical data of ART patients. Because the study was retrospective and was completed through record review, the IRB waived the requirement for informed consent from each participant so that the research could be carried out without contacting patients. The identification (name and unique ART number) of the study participants and healthcare providers who examined each study participant were not included in the data collection instrument to ensure confidentiality. To ensure confidentiality, the data were password-protected after the completion of data entry.
Author Contributions
TGG and FM were responsible for the design of the study. TGG, FM, and TMT conducted the research. TGG and FM supervised data collection. TGG and TMT completed the statistical analyses and drafted the manuscript. TGG and FM contributed to the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
References
1. WHO. HIV and AIDS - Key Facts (2023). Available From https://www.who.int/news-room/fact-sheets/detail/hiv-aids?gclid=CjwKCAjw9-6oBhBaEiwAHv1QvBocOqWgaU_jYq35p7Bj_CqZQYGZ0cnBdNc6jW5UkXjrBRfG0zvDlxoCIlUQAvD_BwE (Accessed July 13, 2023).
2. UNAIDS. Global HIV & AIDS Statistics — 2021 Fact Sheet (2021a). Available From https://www.unaids.org/en/resources/fact-sheet (Accessed February 12, 2023).
3. UNAIDS. Geneva: Joint United Nations Programme on HIV/AIDS (2021b). Available From https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf (Accessed January 02, 2023).
4. UNICEF. Child, HIV, and AIDS-Regional Snapshot-Sub-Saharan Africa (2019). Available From https://reliefweb.int/report/south-africa/children-hiv-and-aids-regional-snapshot-sub-saharan-africa-december-2019 (Accessed February 15, 2023).
5. UNAIDS. HIV and AIDS Estimates Country Factsheet 2020- Ethiopia (2020). Available From https://www.unaids.org/en/regionscountries/countries/ethiopia (Accessed February 12, 2023).
6. Bernstein, L, Brooke, K, Collado, D, Crain, M, Birmingham, A, DiPaolo, C, et al. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection (2001). United States. Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf (Accessed July 10, 2023).
7. Ogunbanjo, G, and Heyer, A. Adherence to HIV Antiretroviral Therapy Part II: Which Interventions Are Effective in Improving Adherence? Open Forum. South Afr Fam Pract (2006) 48(9):6–10. doi:10.1080/20786204.2006.10873455
8. Amico, K, Toro-Alfonso, J, and Fisher, JD. An Empirical Test of the Information, Motivation, and Behavioral Skills Model of Antiretroviral Therapy Adherence. AIDS care (2005) 17(6):661–73. doi:10.1080/09540120500038058
9. Sutton, SS, Magagnoli, J, and Hardin, JW. Odds of Viral Suppression by Single-Tablet Regimens, Multiple-Tablet Regimens, and Adherence Level in HIV/AIDS Patients Receiving Antiretroviral Therapy. Pharmacother J Hum Pharmacol Drug Ther (2017) 37(2):204–13. doi:10.1002/phar.1889
10. Vreeman, RC, Ayaya, SO, Musick, BS, Yiannoutsos, CT, Cohen, CR, Nash, D, et al. Adherence to Antiretroviral Therapy in a Clinical Cohort of HIV-Infected Children in East Africa. PLoS One (2018) 13(2):e0191848. doi:10.1371/journal.pone.0191848
11. Osterberg, L, and Blaschke, T. Adherence to Medication. New Engl J Med (2005) 353(5):487–97. doi:10.1056/NEJMra050100
12. Vreeman, RC, Wiehe, SE, Pearce, EC, and Nyandiko, WM. A Systematic Review of Pediatric Adherence to Antiretroviral Therapy in Low-and Middle-Income Countries. Pediatr Infect Dis J (2008) 27(8):686–91. doi:10.1097/INF.0b013e31816dd325
13. Martin, S, Elliott-DeSorbo, DK, Wolters, PL, Toledo-Tamula, MA, Roby, G, Zeichner, S, et al. Patient, Caregiver, and Regimen Characteristics Associated With Adherence to Highly Active Antiretroviral Therapy Among HIV-Infected Children and Adolescents. Pediatr Infect Dis J (2007) 26(1):61–7. doi:10.1097/01.inf.0000250625.80340.48
14. Mussa, FM, Massawe, HP, Bhalloo, H, Moledina, S, and Assenga, E. Magnitude and Associated Factors of Anti-Retroviral Therapy Adherence Among Children Attending HIV Care and Treatment Clinics in Dar Es Salaam, Tanzania. Plos one (2022) 17(9):e0275420. doi:10.1371/journal.pone.0275420
15. Sutcliffe, CG, van Dijk, JH, Bolton, C, Persaud, D, and Moss, WJ. Effectiveness of Antiretroviral Therapy Among HIV-Infected Children in Sub-Saharan Africa. Lancet Infect Dis (2008) 8(8):477–89. doi:10.1016/S1473-3099(08)70180-4
16. Doyore, F, and Moges, B. Adherence to Antiretroviral Treatment Services and Associated Factors Among Clients Attending ART Clinics in Hosanna Town, Southern Ethiopia. J AIDS Clin Res (2016) 7(590):2. doi:10.4172/2155-6113.1000590
17. Tegegne, D, Mamo, G, Negash, B, Habte, S, Gobena, T, and Letta, S. Poor Adherence to Highly Active Antiretroviral Therapy and Associated Factors Among People Living With HIV in Eastern Ethiopia. SAGE Open Med (2022) 10:20503121221104429. doi:10.1177/20503121221104429
18. PEPFAR. Country/Regional Operational Plan (COP/ROP) 2017: Strategic Direction Summary. East Africa: Ethiopia (2017).
19. Harries, AD, Gomani, P, Teck, R, de Teck, OA, Bakali, E, Zachariah, R, et al. Monitoring the Response to Antiretroviral Therapy in Resource-Poor Settings: The Malawi Model. Trans R Soc Trop Med Hyg (2004) 98(12):695–701. doi:10.1016/j.trstmh.2004.05.002
20. Minn, AC, Kyaw, NTT, Aung, TK, Mon, OM, Htun, T, Oo, MM, et al. Attrition Among HIV Positive Children Enrolled Under Integrated HIV Care Programme in Myanmar: 12 Years Cohort Analysis. Glob Health Action (2018) 11(1):1510593. doi:10.1080/16549716.2018.1510593
21. Nischal, K, Khopkar, U, and Saple, D. Improving Adherence to Antiretroviral Therapy. Indian J Dermatol Venereol Leprol (2005) 71(5):316–20. doi:10.4103/0378-6323.16780
22. Paterson, DL, Swindells, S, Mohr, J, Brester, M, Vergis, E, Squier, C, et al. Adherence to Protease Inhibitor Therapy and Outcomes in Patients With HIV Infection. Ann Intern Med (2000) 133(1):21–30. doi:10.7326/0003-4819-133-1-200007040-00004
23. WHO. Ethiopia FMoHF. National Consolidated Guidelines for Comprehensive HIV Prevention. Care and Treatment. United Kingdom: WHO (2018).
24. Mohammadpour, A, Yekta, ZP, and Nikbakht Nasrabadi, AR. HIV-Infected Patients' Adherence to Highly Active Antiretroviral Therapy: A Phenomenological Study. Nurs Health Sci (2010) 12(4):464–9. doi:10.1111/j.1442-2018.2010.00560.x
25. Carlucci, JG, Liu, Y, Clouse, K, and Vermund, SH. Attrition of HIV-Positive Children From HIV Services in Low-and Middle-Income Countries: A Systematic Review and Meta-Analysis. AIDS (London, England) (2019) 33(15):2375–86. doi:10.1097/QAD.0000000000002366
26. GebreEyesus, F, Mitku, D, Tarekegn, T, Temere, B, Terefe, T, Belete, A, et al. Levels of Adherence and Associated Factors Among Children on ART Over Time in Northwest, Ethiopia: Evidence From a Multicenter Follow-Up Study. HIV/AIDS-Research Palliat Care (2021) 13:829–38. doi:10.2147/HIV.S323090
27. Feyera, B, Letta, S, and Kumie, A. Level of Adherence and Factors Associated With Antiretroviral Therapy Among HIV Infected Children in Selected Public Hospitals, Addis Ababa, Ethiopia. East Afr J Health Biomed Sci (2016) 1(1):23–30.
28. FMOH. National Consolidated Guidelines for Comprehensive HIV Prevention. Care and Treatment (2018).
29. WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. United Kingdom: World Health Organization (2016).
30. Nsheha, AH, Dow, DE, Kapanda, GE, Hamel, BC, and Msuya, LJ. Adherence to Antiretroviral Therapy Among HIV-Infected Children Receiving Care at Kilimanjaro Christian Medical Centre (KCMC), Northern Tanzania: A Cross-Sectional Analytical Study. Pan Afr Med J (2014) 17(1):238. doi:10.11604/pamj.2014.17.238.2280
31. Bhattacharya, M, and Dubey, AP. Adherence to Antiretroviral Therapy and Its Correlates Among HIV-Infected Children at an HIV Clinic in New Delhi. Ann Trop Paediatrics (2011) 31(4):331–7. doi:10.1179/1465328111Y.0000000031
32. Nabukeera-Barungi, N, Kalyesubula, I, Kekitiinwa, A, Byakika-Tusiime, J, and Musoke, P. Adherence to Antiretroviral Therapy in Children Attending Mulago Hospital, Kampala. Ann Trop paediatrics (2007) 27(2):123–31. doi:10.1179/146532807X192499
33. Tiruneh, CM, Emiru, TD, Tibebu, NS, Abate, MW, Nigat, AB, Bantie, B, et al. Clinical Non-adherence and Its Associated Factors Among HIV-Positive Pediatric Patients Attending HIV Care in South Gondar Zone Public Health Facilities, Northwest Ethiopia 2021. HIV/AIDS - Res Palliat Care (2022) 14:23–32. doi:10.2147/HIV.S352386
34. Alemu, K, Likisa, J, Alebachew, M, Temesgen, G, Tesfaye, G, and Dinsa, H. Adherence to Highly Active Antiretroviral Therapy and Predictors of Non-Adherence Among Pediatrics Attending Ambo Hospital ART Clinic, West Ethiopia. J HIV AIDS Infect Dis (2014) 2:1–7.
35. Mehta, K, Ekstrand, M, Heylen, E, Sanjeeva, G, and Shet, A. Adherence to Antiretroviral Therapy Among Children Living With HIV in South India. AIDS Behav (2016) 20:1076–83. doi:10.1007/s10461-015-1207-7
36. Iroha, E, Esezobor, CI, Ezeaka, C, Temiye, EO, and Akinsulie, A. Adherence to Antiretroviral Therapy Among HIV-Infected Children Attending a Donor-Funded Clinic at a Tertiary Hospital in Nigeria. Afr J AIDS Res (2010) 9(1):25–30. doi:10.2989/16085906.2010.484543
37. Desta, AA, Kidane, KM, Woldegebriel, AG, Ajemu, KF, Berhe, AA, Zgita, DN, et al. Level of Adherence and Associated Factors Among HIV-Infected Patients on Antiretroviral Therapy in Northern Ethiopia: Retrospective Analysis. Patient preference and adherence (2020) 14:1585–94. doi:10.2147/PPA.S268395
38. Endalamaw, A, Tezera, N, Eshetie, S, Ambachew, S, and Habtewold, TD. Adherence to Highly Active Antiretroviral Therapy Among Children in Ethiopia: A Systematic Review and Meta-Analysis. AIDS Behav (2018) 22(8):2513–23. doi:10.1007/s10461-018-2152-z
39. Gultie, T, and Sebsibie, G. Factors Affecting Adherence to Pediatrics Antiretroviral Therapy in Mekelle Hospital, Tigray Ethiopia. Int J Public Health Sci (2015) 4(1):1–6. doi:10.11591/.v4i1.4704
40. Teshome, W, Belayneh, M, Moges, M, Endriyas, M, Mekonnen, E, Ayele, S, et al. Who Takes the Medicine? Adherence to Antiretroviral Therapy in Southern Ethiopia. Patient preference and adherence (2015) 9:1531–7. doi:10.2147/PPA.S90816
41. FMOH. Guidelines for Paediatric HIV/AIDS Care and Treatment in Ethiopia. Ethiopia: HIV/AIDS Prevention and Control Office Addis Ababa (2008).
Keywords: antiretroviral therapy, children, HIV, suboptimal adherence, Ethiopia
Citation: Guyo TG, Merid F and Toma TM (2023) Predictors of Suboptimal Adherence Among Children on Antiretroviral Therapy in Southern Ethiopia: A Multicenter Retrospective Follow-Up Study. Int J Public Health 68:1606520. doi: 10.3389/ijph.2023.1606520
Received: 16 August 2023; Accepted: 26 October 2023;
Published: 09 November 2023.
Edited by:
Lyda Osorio, University of the Valley, ColombiaReviewed by:
Isabel Hurtado, University of the Valley, ColombiaRajendra Gadhavi, B. J. Medical College and Civil hospital, India
Copyright © 2023 Guyo, Merid and Toma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tamirat Gezahegn Guyo, dGFtaXJhdGdlemFoZWduN3N0QGdtYWlsLmNvbQ==
†ORCID: Tamirat Gezahegn Guyo, orcid.org/0000-0001-7084-3570; Fasika Merid, orcid.org/0000-0002-4080-2724; Temesgen Mohammed Toma, orcid.org/0000-0001-8849-6722
This Original Article is part of the IJPH Special Issue “Hunger, Food Sovereignty & Public Health”
‡These authors have contributed equally to this work
 Tamirat Gezahegn Guyo
Tamirat Gezahegn Guyo