Abstract
Objectives: Sleep is a conserved vital behavior in humans, and insufficient sleep is associated with several disorders. Recent studies have investigated the association of sleep duration, oxidative stress markers, anxiety, and depression. Therefore, we aim to assess the relationship between sleep duration, serum pro-oxidant/antioxidant balance (PAB) and superoxide dismutase 1 (SOD1) levels as markers of oxidative stress, anxiety, and depression.
Methods: Participants included in our cross-sectional analysis were recruited as part of the MASHAD study (n = 9,184). Nocturnal sleep duration was identified using a self-reported questionnaire, and serum pro-oxidant/antioxidant balance (PAB) and superoxide dismutase 1 (SOD1) levels were assessed using methods that have been previously reported.
Results: Serum PAB, depression, and anxiety scores were found significantly higher in subjects with very short sleep duration. In an adjusted model using MANOVA regression analysis, serum PAB was significantly higher in the subjects with a very short sleep duration (p: 0.016 in depression and p: 0.002 in anxiety).
Conclusion: The present cross-sectional study demonstrates a relationship between sleep duration, oxidative balance, and depression/anxiety, especially in anxiety subjects that might predict each other.
Introduction
Sleep deprivation refers to a state in which an individual gets less sleep than required to feel alert and awake [1]. It has been reported that 82.6% of retired Iranian elders suffer from poor sleep quality [2]. Moreover, from 2003 to 2012, sleep insufficiency was consistently increasing in children subjected to the National Survey of Children’s Health [3]. In support of the physiological importance of sleep, many studies have indicated that sleep deprivation is associated with several conditions, including psychiatric disorders, cardiovascular diseases, inflammation, metabolic disorders, immune dysfunction, and even premature death [4–9]. Furthermore, it is the leading cause of car accidents in Iran, accounting for the death of 20,000–80,000 individuals in Iran annually [10, 11]. Another recently proposed hypothesis for the adverse effects of sleep deprivation is the disturbance of pro-oxidant/antioxidant balance (PAB) in favor of oxidants, which leads to oxidative stress [12]. One study suggested that the superoxide dismutase1 (SOD1) enzyme, which is the first defense line against superoxide free radicals and also the most extensively studied antioxidant enzyme, is disrupted in individuals with sleep deprivation, which leads to oxidative stress (OS) [13]. Epidemiological studies have also suggested an association between sleep deprivation and affective disorders such as depression and anxiety in children and adolescents [14, 15]. Another study has suggested that early life sleep deprivation was a predictive risk factor for later onset of anxiety and depression [16]. According to the World Health Organization report in 2015, more than 322 and 264 million people are struggling with depression and anxiety disorders worldwide, making up 4.4% and 3.6% of the global population [17]. A systematic review showed that prevalence of depression and anxiety increased to 279.6 and 301.4 million people [18]. Moreover, the incidence of these two common psychiatric disorders is increasing, especially in countries with low or middle income [19]. Depression is the single largest, and anxiety is the sixth most significant cause of disability and health loss globally [19].
To date, there has been no consensus on the physiological functions of sleep. A controversial hypothesis for the physiological role of sleep is acting as an antioxidant mechanism for the brain, which was proposed by Reimund [20]. The Reactive Oxygen Species (ROS) and oxidative stress markers could be accumulated in the brain tissue during Sleep deprivation [21]. Some stud suggested, enough sleep can increase antioxidant activity which promotes a brain protection against free radicals via a decrease in oxidant production [22]. Oxidative stress can also result in a wide range of diseases such as neurodegenerative diseases, cancer, male infertility, diabetes, autoimmune diseases, and cardiovascular disorders [4, 23–27]. In the past few years, numerous studies have sought to evaluate the involvement of OS in psychiatric disorders. Predominantly, therapeutic methods for depression and anxiety have focused on gamma-aminobutyric acidergic and serotoninergic systems [28]. But, researchers have established a link between OS and depression/anxiety during the last few years, suggesting that other systems like oxidative metabolism can affect mental health, which might draw more attention to antioxidants [28–30]. Nonetheless, there remains a controversy surrounding the association of depression/anxiety with PAB. It is not yet well understood whether depression/anxiety are associated with increased or decreased PAB [31].
Understanding the physiological functions of sleep and its role in antioxidant mechanisms is necessary for identifying the impacts of sleep deprivation on mental health. Since most studies regarding the effect of sleep deprivation on PAB and anxiety/depression and the association between serum PAB and anxiety/depression are mostly conducted on animals or non-population-based with limited sample sizes, we cannot have a decisive conclusion. Consequently, the primary objective of this population-based cross-sectional study was to investigate the impact of sleep duration on PAB, SOD1, anxiety, and depression. The secondary objective was to evaluate the association of these oxidative stress determinants with anxiety and depression in subjects with very short nightly sleep (Figure 1).
FIGURE 1
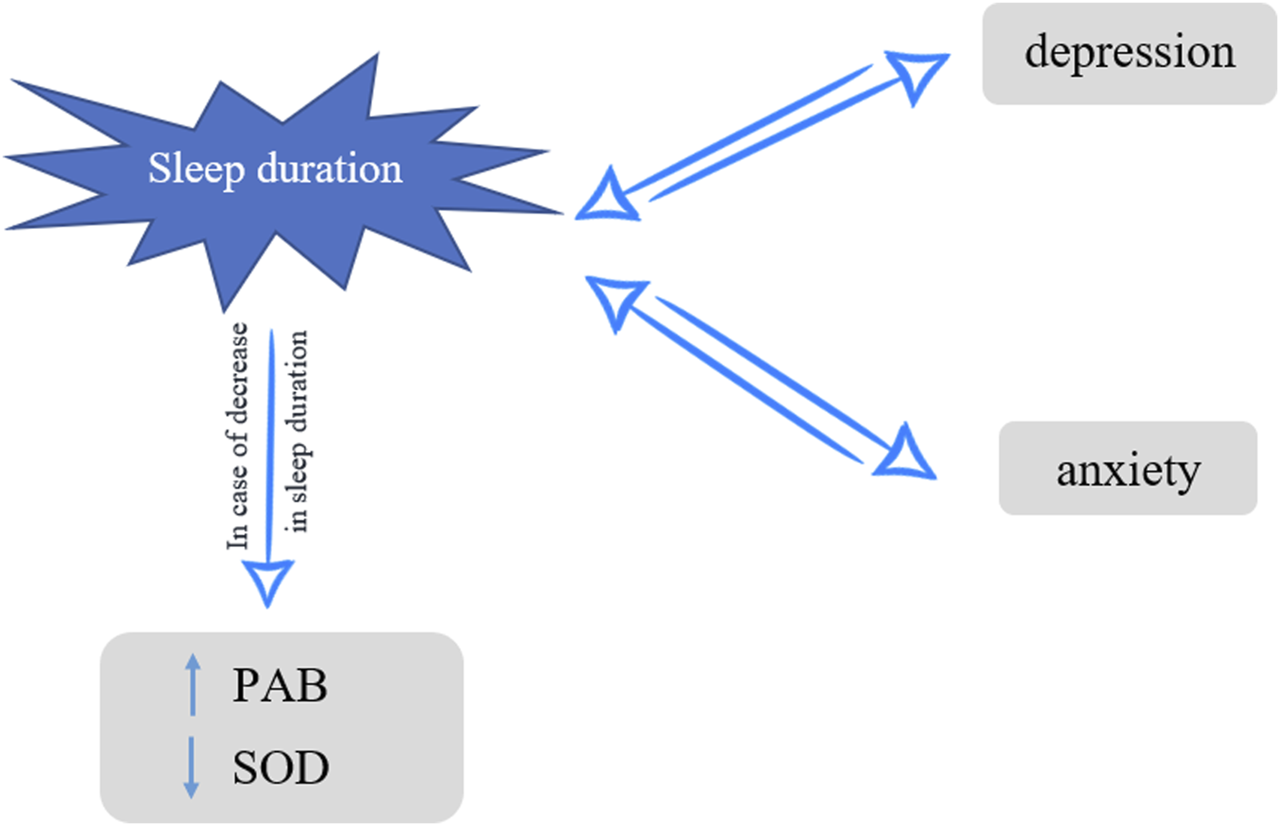
Nightly sleep, oxidative stress and depression/anxiety, Mashhad stroke and heart atherosclerotic disorders, Iran, 2010–2020.
Methods
Study Population
This research study was conducted on subjects recruited as part of the Mashhad stroke and heart atherosclerotic disorders (MASHAD) 10 year cohort study, which was started in 2010 and is continued up to 2020 on 9,704 Mashhad citizens selected from three regions of Mashhad, a highly populated city in the northeast of Iran. MASHAD study characteristics and methodology was discussed in detail in the previously published manuscript [32]. The inclusion criteria was age 35–65 and no history of CVD. The exclusion criteria for recruitment at baseline including no history of CHD, cancer, chronic kidney disease.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of the Mashhad University of Medical Sciences (MUMS) (MASHAD study code: 85134). All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all subjects in the ethics approval and consent to participate. The ethic approval was IR.MUMS.REC.1386.250.
Sleep Measurement
We assessed the nocturnal sleep duration of our participants using a questionnaire (self-report). Thereafter, subjects were put in three defined subgroups: <5 (very short sleep), 5–7 h (short sleep), and ≥7 h (normal and long sleep). Night shift workers and subjects in the very short sleep (<5 h) subgroups who sleep in the afternoons were not included in this cross-sectional analysis [33].
Serum PAB Measurement
Measuring all oxidants and antioxidants separately is costly and time-consuming. The PAB assay, developed by Hamidi et al., is a technique that simultaneously measures oxidants and antioxidants in a single test [34]. A standard curve was provided by the values determined in the standard samples. The PAB values were expressed in arbitrary HK (Hamidi-Koliakos) unit, which are the percentage of hydrogen peroxide in the standard solution. Finally, the values of the unknown samples were calculated based on the values obtained from the standard curve.
Serum SOD1 Assay
The activity of superoxide dismutase-1 activity was assessed by pyrogallol solution and Tris–cacodylic acid buffer under the conditions explained [35].
Depression and Anxiety Assessment
For measuring depression in all participants, Beck Depression Inventory (BDI) [36] questionnaire has been applied in which consisted of 21 items, and each item was scored from 0 to 3. Based on the questionnaire results, all subjects were scored from 0 to 63, and scores in 0–13, 14–19, 20–28, and 29–63 were considered and categorized into minimal, mild, moderate, and severe depression subgroups, respectively [37]. Furthermore, for assessing anxiety symptoms, we used Beck Anxiety Inventory (BAI) [38]. This questionnaire was mainly used to evaluate anxiety symptoms frequency, but due to previous results, it was utilized to identify the severity of anxiety in this study subjects [39]. The same as BDI, the BAI questionnaire had 21 items with a score of 0–3 for each item, and totally 0 to 63 points were given to each subject. Participants with a score of 0–7, 8–15, 16–25, and 26–63 were divided into minimal, mild, moderate, and severe anxiety subgroups, respectively [38]. Previously, the Persian version of BDI and BAI questionnaires was validated appropriately by Ghassemzadeh et al. [40] and Kaviani and Mousavi [41] studies, respectively.
Statistical Analysis
Data were analyzed using two-sided tests using SPSS version 23 (SPSS Inc. Released 2009. PASW Statistics for Windows, Version 23.0. Chicago: SPSS Inc.). Quantitative and qualitative variables are reported as mean ± SD and number (percentage), respectively. Kolmogorov-Smirnov analytical test was performed to check whether the data are normally distributed or not. Chi-square analysis was performed in order to assess differences between subgroups of two qualitative variables, and we used a one-way analysis of variance (ANOVA) test for the determination of differences in the amount of normally-distributed quantitative variables according to subgroups of nightly sleep duration or anxiety or depression severities. Moreover, we applied the multivariate analysis of variance (MANOVA) test to evaluate changes in the serum level of PAB and SOD1 in the subgroups of nightly sleep duration. We defined p-value under 0.05 as significant. Graph pad prism was used for drawing figures.
Results
In the current analysis, 9,184 subjects were eligible and enrolled, of whom 3,682 (40.09%) were male. As indicated in Table 1, a chi-square analysis found men were significantly more likely to experience short sleep duration than women. In addition, one-way ANOVA indicated a significant difference in age, depression and anxiety scores, and serum PAB level between three defined subgroups of nightly sleep duration. After performing post hoc analysis, we found that anxiety and depression scores are meaningfully higher in very short sleep subjects in comparison with the other groups. Furthermore, for PAB, it was considerably higher in the very short sleep subgroup than just normal subjects. No significant differences were found in all mentioned features between short and normal participants.
TABLE 1
| Very short sleep <5 h | Short sleep 5–7 h | Normal sleep >7 h | p-value | |
|---|---|---|---|---|
| Number | N = 456, 4.96% | N = 2,968, 32.31% | N = 5,760, 62.71% | |
| Age, years | 51.27 ± 7.76 | 48.99 ± 7.97 | 47.25 ± 7.96 | <0.001 |
| Sex, N (%) | ||||
| Male | 176 (4.78) | 1,248 (33.89)* | 2,258 (61.32) | 0.029 |
| Female | 280 (5.08) | 1,720 (31.26) | 3,502 (63.64) | |
| Depression score | 16.67 ± 11.93 | 13.17 ± 9.85a | 11.77 ± 9.26a | <0.001 |
| Anxiety score | 15.74 ± 12.84 | 11.31 ± 10.08a | 9.85 ± 9.24a | <0.001 |
| SOD1, IU | 2.22 ± 1.86 | 2.15 ± 1.8 | 2.12 ± 1.78 | 0.46 |
| PAB, HK | 72.16 ± 58.51 | 68.76 ± 53.46 | 65.77 ± 55.23a | 0.001 |
Study participants’ demographic characteristics and oxidative stress determinants based on their nightly sleep duration in Mashhad stroke and heart atherosclerotic disorders, Iran, 2010–2020.
*significant differences is related to this group. a: <5 h vs. 5–7 h and >7 h. Chi-square test has been done for evaluating differences of sex distribution in subgroups of nightly sleep duration and one-way ANOVA was performed for analysis of the other variables; Mean and Standard Deviation (SD) of the quantitative data in the target population is represented as Mean ± SD; PAB, pro-oxidant Antioxidant Balance; SOD1, superoxide dismutase 1.
Based on results showed in Figure 2, in participants with very short nightly sleep, severe cases were found significantly more frequent depression and anxiety than the other groups. Moreover, as shown in Figure 3, the nightly sleep duration and serum PAB level were respectively, significantly lower and higher in severe depressive and anxiety cases than the other ones.
FIGURE 2
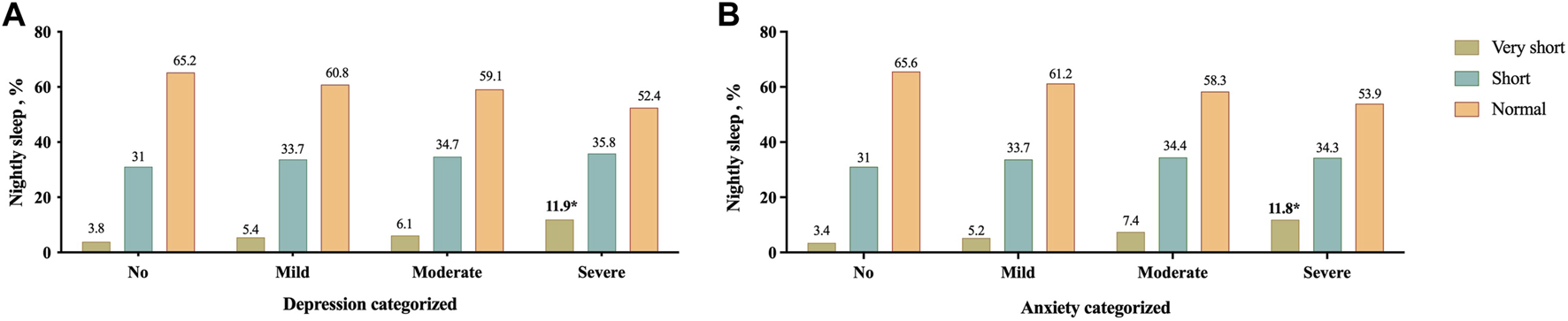
Prevalence of different severity categories of depression (A) and anxiety (B) based on their nightly sleep duration; *p < 0.05, Mashhad stroke and heart atherosclerotic disorders, Iran, 2010–2020.
FIGURE 3
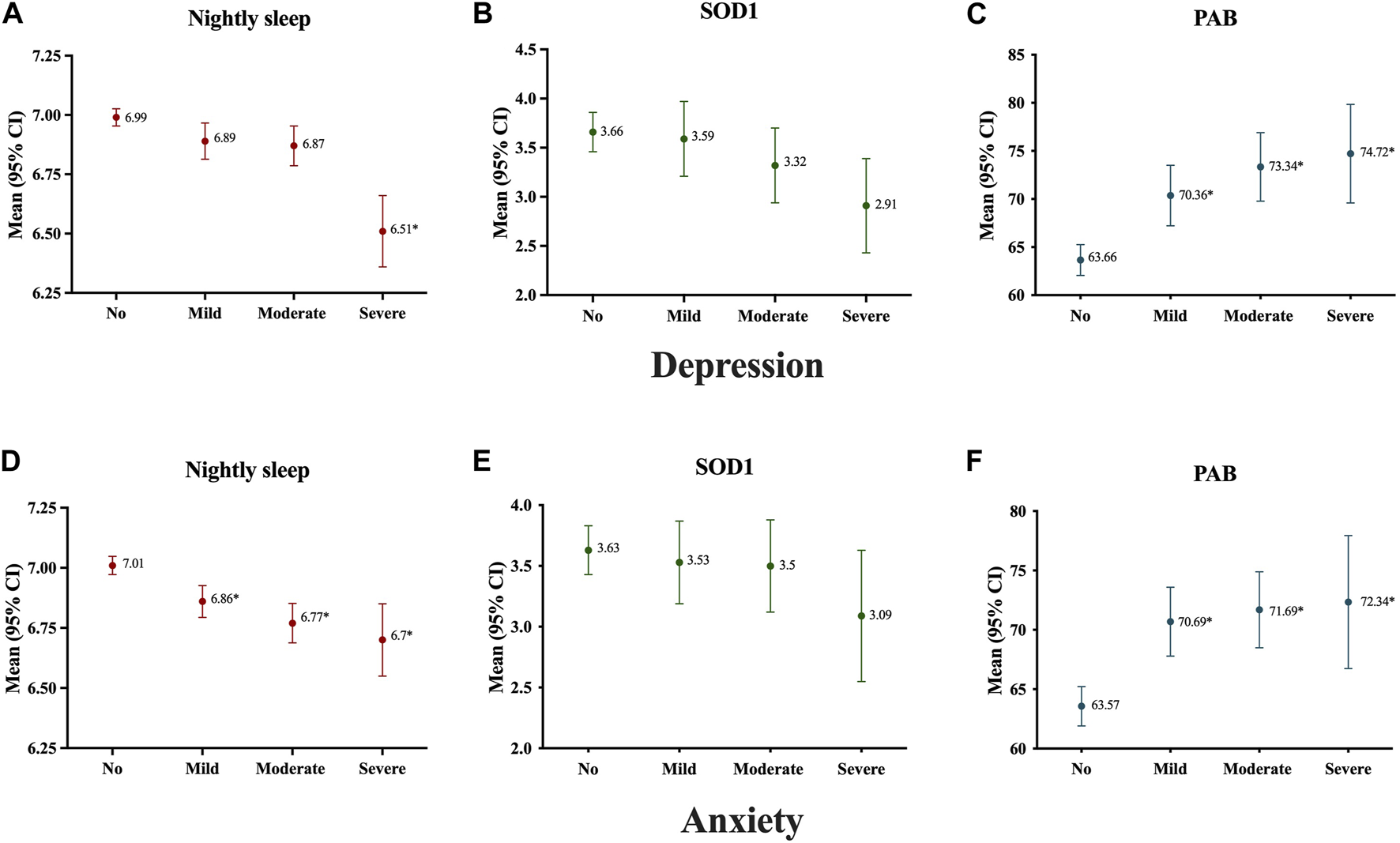
Antioxidant status and nightly sleep duration of study population according to severity of subjects’ depression (A–C) and anxiety (D–F); Data presented as mean ± 2SE; *p < 0.05, Mashhad stroke and heart atherosclerotic disorders, Iran, 2010–2020.
In crude analysis, it was found that in both depressive and anxiety subjects, serum PAB was significantly higher in very short sleep subjects in comparison with the others, respectively (Figure 4). After adjusting results for age and sex in a MANOVA regression analysis, we found that subjects with anxiety and depression and very short sleep had 12.09- and 10.69-unit PAB more than others, respectively (Table 2).
FIGURE 4
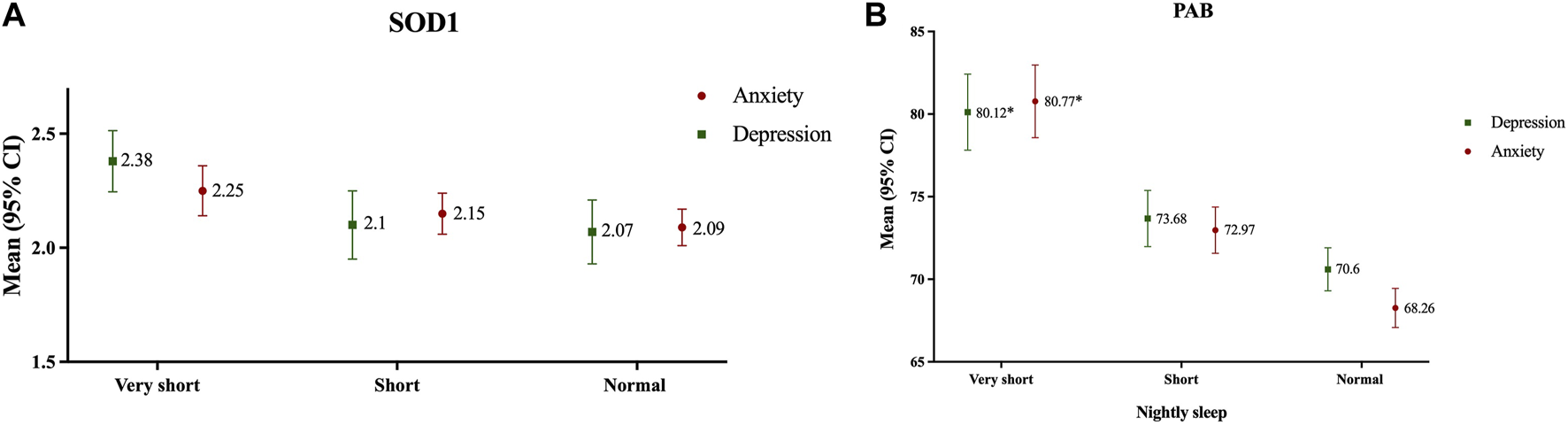
Antioxidant status according to nightly sleep duration in subjects with depression and anxiety [(A): SOD; (B): PAB]; Data presented as mean ± 2SE; *p < 0.05, Mashhad stroke and heart atherosclerotic disorders, Iran, 2010–2020.
TABLE 2
| Depression subjects | ||||
|---|---|---|---|---|
| Nightly sleep duration | Beta coefficient | SE | p-value | |
| Serum PAB | >7 h (Ref.) | |||
| 5–7 h | 3.822 | 2.264 | 0.092 | |
| <5 h | 10.696 | 4.456 | 0.016 | |
| Serum SOD1 | >7 h (Ref.) | |||
| 5–7 h | 0.026 | 0.069 | 0.75 | |
| <5 h | 0.31 | 0.128 | 0.15 | |
| Anxiety subjects | ||||
| Serum PAB | >7 h (Ref.) | |||
| 5–7 h | 3.921 | 1.93 | 0.042 | |
| <5 h | 12.092 | 3.882 | 0.002 | |
| Serum SOD1 | >7 h (Ref.) | |||
| 5–7 h | 0.45 | 0.073 | 0.42 | |
| <5 h | 0.12 | 0.151 | 0.54 | |
Impact of nightly sleep duration on serum level of PAB and SOD1 in subjects with depression and anxiety separately, respectively adjusted for age and sex in Mashhad stroke and heart atherosclerotic disorders, Iran, 2010–2020.
Multivariate Analysis of Variance (MANOVA) test was performed; SE, standard error.
Discussion
We have demonstrated that very short nightly sleep was significantly associated with higher depression and anxiety scores. There was a significant association between sleep duration and serum PAB level, but serum SOD1 activity was not associated with sleep duration. In individuals with Generalized Anxiety Disorder (GAD), sleep duration was conversely associated with PAB. In other words, the less sleep duration, the higher PAB.
In 1994, Reimund suggested that a key function of sleep might be acting as an antioxidant for the brain [20]. Oxidative phosphorylation -taking place in mitochondria-is the main ATP supply of the cell in all aerobic organisms that produce free radicals as a by-product [31]. At low concentrations, free radicals are necessary for normal cell functioning. They serve numerous physiological processes, including mitosis, gametogenesis, cell signaling, and cellular response to infection and injury [25, 42]. When the disturbance in pro-oxidant/antioxidant balance (PAB) occurs in favor of the oxidants, cells may be exposed to oxidative stress. OS results from either increased generation of reactive oxygen species (ROS) or impaired oxidative defense mechanisms. SOD is the most extensively studied antioxidant enzyme and serves as the first defense line against superoxide free radicals, which catalyzes the dismutation of the highly reactive superoxide radical, O2−, to O2 and H2O2 [13, 31]. The brain is especially more susceptible to OS because it is mostly made up of lipid and consumes comparatively large amounts of oxygen and produces an excessive amount of free radicals with a relatively modest oxidative defense system [43]. In support of the Reimund hypothesis, Hill and associates have indicated that the upregulation of gene expression of antioxidant enzymes in wild-type flies shortens sleep duration, suggesting that decreasing PAB promotes wakefulness [44]. One previous study has reported that sleep deprivation may induce oxidative stress, suggesting that sleep clears the ROS accumulated in the brain during wakefulness [45].
We found that inadequate sleep is associated with a high serum PAB in subjects with anxiety and depression. To our knowledge, we are the first study to show this association. In contrast to our findings, in studies conducted on humans, OS was not observed in sleep-deprived individuals [46–48]. Furthermore, in a narrative review, Atrooz et al. have suggested that while chronic sleep deprivation diminishes the antioxidant response, acute sleep deprivation may augment antioxidant mechanisms [13]. There are inconsistent results regarding the impact of sleep deprivation on SOD1 activity. In line with our findings, Gopalakrishnan and coworkers found no significant change in SOD1 activity in rats with chronic sleep deprivation [46].
As previously discussed, the brain is especially vulnerable to oxidative stress (OS); and OS can affect synaptic integrity, neurotransmission, and the overall function of the brain, resulting in neurological and psychological disorders [49, 50]. Data on the relationship between OS and depression/anxiety are controversial. In line with our findings, some authors have revealed a strong positive relationship between OS and depression/anxiety [28, 51, 52]. Contrastingly, some authors have failed to demonstrate an association between OS and the presence of depression/anxiety [53–55]. When it comes to the activity of antioxidant enzymes in depression/anxiety, the data are discrepant. While we found no differences in serum SOD1 activity in different depression/anxiety severities, similarly, as observed in another study, both increased and decreased activity of SOD1 have been reported previously [31, 56, 57]. An increased expression of SOD1 may induce inflammation, which inflammation itself can contribute to depression/anxiety [29, 58, 59]. Far as we know, researches evaluating the association between different depression/anxiety severities and OS using PAB are scarce. In contrast with our results, in another study, no association was found between serum PAB level and severity of depression [55].
Our study has assessed the trinary relationship between subjects’ sleep duration, oxidative stress, and anxiety/depression scores of a large randomly-selected population in a city in the Middle East. In addition to our considerable efforts, some limitations existed in our procedures. First, our analysis was a cross-sectional one that could not accurately conclude cause and effect relationships between these three mentioned features. Moreover, data on the nocturnal sleep duration of our participants were collected using self-report questionnaires and we could not assess other oxidative stress parameters. Further cohort and case-controlled studies with more accurate methods for observation of sleep are required to better identify the risks of sleep deprivation.
In conclusion, our cross-sectional analysis revealed a logical trinary association between sleep duration, oxidative stress, and anxiety/depression and mainly indicated that sleep deprivation is associated with increased pro-oxidant/antioxidant balance and severe depression/anxiety. For future works we can use another categorization for response variables and also we can use machine learning algorithms for analyzing the data.
Statements
Data availability statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by the Mashhad university of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SuD: data analysis, data gathering. ME, SiD, and KJA-F: wrote manuscript. HE: data analysis and study design. AH, MZ, and RD: data gathering. GF: scientific and grammatical editing. MM: corresponding author, designed the study. MG-M: designed the study.
Funding
This work was supported by the Mashhad University of Medical Sciences (grant number 85134).
Acknowledgments
Mashhad University of Medical Sciences.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
References
1.
NHLBI. National Heart, Lung, and Blood Institute (2022). Available From: https://www.nhlbi.nih.gov/health-topics/sleep-deprivation-and-deficiency. (Accessed 24 March 2022).
2.
Malakouti SK Foroughan M Nojomi M Ghalebandi MF Zandi T . Sleep Patterns, Sleep Disturbances and Sleepiness in Retired Iranian Elders. Int J Geriatr Psychiatry (2009) 24(11):1201–8. 10.1002/gps.2246
3.
Hawkins SS Takeuchi DT . Social Determinants of Inadequate Sleep in US Children and Adolescents. Public Health (2016) 138:119–26. 10.1016/j.puhe.2016.03.036
4.
Knutson KL Spiegel K Penev P Van Cauter E . The Metabolic Consequences of Sleep Deprivation. Sleep Med Rev (2007) 11(3):163–78. 10.1016/j.smrv.2007.01.002
5.
Shamsuzzaman AS Gersh BJ Somers VK . Obstructive Sleep Apnea: Implications for Cardiac and Vascular Disease. Jama (2003) 290(14):1906–14. 10.1001/jama.290.14.1906
6.
Goldstein AN Walker MP . The Role of Sleep in Emotional Brain Function. Annu Rev Clin Psychol (2014) 10:679–708. 10.1146/annurev-clinpsy-032813-153716
7.
van Leeuwen WM Lehto M Karisola P Lindholm H Luukkonen R Sallinen M et al Sleep Restriction Increases the Risk of Developing Cardiovascular Diseases by Augmenting Proinflammatory Responses Through IL-17 and CRP. PLoS One (2009) 4(2):e4589. 10.1371/journal.pone.0004589
8.
Irwin MR Wang M Campomayor CO Collado-Hidalgo A Cole S . Sleep Deprivation and Activation of Morning Levels of Cellular and Genomic Markers of Inflammation. Arch Intern Med (2006) 166(16):1756–62. 10.1001/archinte.166.16.1756
9.
Gallicchio L Kalesan B . Sleep Duration and Mortality: A Systematic Review and Meta-Analysis. J Sleep Res (2009) 18(2):148–58. 10.1111/j.1365-2869.2008.00732.x
10.
Abbasi M Sadeghi M Azami AA Esmaeili SM Kavousi J Aryafard A . Factors Related to Road Traffic Accidents Leading to Injury or Death in. Shahroud City (2016) 4(2):8. 10.30491/TM.2020.213470.1007
11.
Fu X Wang H Zhang X . Genetic Aspects of Early Menopause. J Bio-X Res (2019) 2(3):105–11. 10.1097/jbr.0000000000000043
12.
Teixeira KRC dos Santos CP de Medeiros LA Mendes JA Cunha TM De Angelis K et al Night Workers Have Lower Levels of Antioxidant Defenses and Higher Levels of Oxidative Stress Damage When Compared to Day Workers. Scientific Rep (2019) 9(1):4455. 10.1038/s41598-019-40989-6
13.
Atrooz F . Salim S: Sleep Deprivation, Oxidative Stress and Inflammation. Adv Protein Chem Struct Biol (2020) 119:309–36. 10.1016/bs.apcsb.2019.03.001
14.
Pires GN Bezerra AG Tufik S Andersen ML . Effects of Acute Sleep Deprivation on State Anxiety Levels: A Systematic Review and Meta-Analysis. Sleep Med (2016) 24:109–18. 10.1016/j.sleep.2016.07.019
15.
Roberts RE Duong HT . The Prospective Association Between Sleep Deprivation and Depression Among Adolescents. Sleep (2014) 37(2):239–44. 10.5665/sleep.3388
16.
Gregory AM Caspi A Eley TC Moffitt TE Oconnor TG Poulton R . Prospective Longitudinal Associations Between Persistent Sleep Problems in Childhood and Anxiety and Depression Disorders in Adulthood. J Abnorm Child Psychol (2005) 33(2):157–63. 10.1007/s10802-005-1824-0
17.
World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. Geneva, Switzerland: World Health Organization (2017).
18.
GBD MentalDisorders Collaborators. Global, Regional, and National Burden of 12 Mental Disorders in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. The Lancet Psychiatry (2022) 9(2):137–50. 10.1016/S2215-0366(21)00395-3
19.
Muka T Oliver-Williams C Kunutsor S Laven JSE Fauser BCJM Chowdhury R et al Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-Analysis. JAMA Cardiol (2016) 1(7):767–76. 10.1001/jamacardio.2016.2415
20.
Reimund E . The Free Radical Flux Theory of Sleep. Med Hypotheses (1994) 43(4):231–3. 10.1016/0306-9877(94)90071-x
21.
Palagini L Geoffroy PA Miniati M Perugi G Biggio G Marazziti D et al Insomnia, Sleep Loss, and Circadian Sleep Disturbances in Mood Disorders: A Pathway Toward Neurodegeneration and Neuroprogression? A Theoretical Review. CNS spectrums (2022) 27(3):298–308. 10.1017/S1092852921000018
22.
Besedovsky L Lange T Haack M . The Sleep-Immune Crosstalk in Health and Disease. Physiol Rev (2019) 99:1325–80. 10.1152/physrev.00010.2018
23.
Duffy SL Lagopoulos J Cockayne N Hermens DF Hickie IB Naismith SL . Oxidative Stress and Depressive Symptoms in Older Adults: A Magnetic Resonance Spectroscopy Study. J Affect Disord (2015) 180:29–35. 10.1016/j.jad.2015.03.007
24.
Noguti J Andersen ML Cirelli C Ribeiro DA . Oxidative Stress, Cancer, and Sleep Deprivation: Is There a Logical Link in This Association?Sleep Breath (2013) 17(3):905–10. 10.1007/s11325-012-0797-9
25.
Tvrda E Massányi P Lukac N . Physiological and Pathological Roles of Free Radicals in Male Reproduction. London, UK: IntechOpen (2018).
26.
Hsiao YH Chen YT Tseng CM Wu LA Lin WC Su VY et al Sleep Disorders and Increased Risk of Autoimmune Diseases in Individuals Without Sleep Apnea. Sleep (2015) 38(4):581–6. 10.5665/sleep.4574
27.
Tobaldini E Costantino G Solbiati M Cogliati C Kara T Nobili L et al Sleep, Sleep Deprivation, Autonomic Nervous System and Cardiovascular Diseases. Neurosci Biobehav Rev (2017) 74:321–9. 10.1016/j.neubiorev.2016.07.004
28.
Bouayed J Rammal H Soulimani R . Oxidative Stress and Anxiety Relationship and Cellular Pathways. Oxidative Med Cell Longevity (2009) 2(2):63–7. 10.4161/oxim.2.2.7944
29.
Salim S . Oxidative Stress and Psychological Disorders. Curr Neuropharmacol (2014) 12(2):140–7. 10.2174/1570159X11666131120230309
30.
Smaga I Niedzielska E Gawlik M Moniczewski A Krzek J Przegaliński E et al Oxidative Stress as an Etiological Factor and a Potential Treatment Target of Psychiatric Disorders. Part 2. Depression, Anxiety, Schizophrenia and Autism. Pharmacol Rep (2015) 67(3):569–80. 10.1016/j.pharep.2014.12.015
31.
Hovatta I Juhila J Donner J . Oxidative Stress in Anxiety and Comorbid Disorders. Neurosci Res (2010) 68(4):261–75. 10.1016/j.neures.2010.08.007
32.
Ghayour-Mobarhan M Moohebati M Esmaily H Ebrahimi M Parizadeh SMR Heidari-Bakavoli AR et al Mashhad Stroke and Heart Atherosclerotic Disorder (MASHAD) Study: Design, Baseline Characteristics and 10-Year Cardiovascular Risk Estimation. Int J Public Health (2015) 60(5):561–72. 10.1007/s00038-015-0679-6
33.
Darroudi S Sharifan P Sadeghzadeh P Namjou N Bidary MZ Zamani P et al Overweight and Obesity are Potential Risk Factors for Disrupted Nocturnal Sleep in Iranian Adults: A Cross-Sectional Study. Int J Public Health (2021) 66:633183. 10.3389/ijph.2021.633183
34.
Alamdari DH Paletas K Pegiou T Sarigianni M Befani C Koliakos G . A Novel Assay for the Evaluation of the Prooxidant–Antioxidant Balance, Before and After Antioxidant Vitamin Administration in Type II Diabetes Patients. Clin Biochem (2007) 40(3):248–54. 10.1016/j.clinbiochem.2006.10.017
35.
Darroudi S Fereydouni N Tayefi M Ahmadnezhad M Zamani P Tayefi B et al Oxidative Stress and Inflammation, Two Features Associated With a High Percentage Body Fat, and That May Lead to Diabetes Mellitus and Metabolic Syndrome. BioFactors (2019) 45(1):35–42. 10.1002/biof.1459
36.
Dozois DJA Dobson KS Ahnberg JL . A Psychometric Evaluation of the Beck Depression Inventory–II. Psychol Assess (1998) 10(2):83–9. 10.1037/1040-3590.10.2.83
37.
Scogin F Beutler L Corbishley A Hamblin D . Reliability and Validity of the Short Form Beck Depression Inventory With Older Adults. J Clin Psychol (1988) 44(6):853–7. 10.1002/1097-4679(198811)44:6<853:aid-jclp2270440604>3.0.co;2-7
38.
Beck AT Epstein N Brown G Steer RA . An Inventory for Measuring Clinical Anxiety: Psychometric Properties. J Consulting Clin Psychol (1988) 56(6):893–7. 10.1037//0022-006x.56.6.893
39.
Muntingh ADT van der Feltz-Cornelis CM van Marwijk HWJ Spinhoven P Penninx BWJH van Balkom AJLM . Is the Beck Anxiety Inventory a Good Tool to Assess the Severity of Anxiety? A Primary Care Study in The Netherlands Study of Depression and Anxiety (NESDA). BMC Fam Pract (2011) 12(1):66. 10.1186/1471-2296-12-66
40.
Ghassemzadeh H Mojtabai R Karamghadiri N Ebrahimkhani N . Psychometric Properties of a Persian-Language Version of the Beck Depression Inventory-Second Edition: BDI-II-PERSIAN. Depress Anxiety (2005) 21(4):185–92. 10.1002/da.20070
41.
Hossein Kaviani H Mousavi AS . Psychometric Properties of the Persian Version of Beck Anxiety Inventory (Bai). Tehran Univ Med J (2008) 66(2):136–40.
42.
Valko M Rhodes CJ Moncol J Izakovic M Mazur M . Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem Biol Interact (2006) 160(1):1–40. 10.1016/j.cbi.2005.12.009
43.
Halliwell B . Oxidative Stress and Neurodegeneration: Where Are We Now?J Neurochem (2006) 97(6):1634–58. 10.1111/j.1471-4159.2006.03907.x
44.
Hill VM O'Connor RM Sissoko GB Irobunda IS Leong S Canman JC et al A Bidirectional Relationship Between Sleep and Oxidative Stress in Drosophila. Plos Biol (2018) 16(7):e2005206. 10.1371/journal.pbio.2005206
45.
Lira AB de Sousa Rodrigues CF . Evaluation of Oxidative Stress Markers in Obstructive Sleep Apnea Syndrome and Additional Antioxidant Therapy: A Review Article. Sleep Breath (2016) 20(4):1155–60. 10.1007/s11325-016-1367-3
46.
Gopalakrishnan A Ji LL Cirelli C . Sleep Deprivation and Cellular Responses to Oxidative Stress. Sleep (2004) 27(1):27–35. 10.1093/sleep/27.1.27
47.
D'Almeida V Hipólide DC Azzalis LA Lobo LL Junqueira VB Tufik S . Absence of Oxidative Stress Following Paradoxical Sleep Deprivation in Rats. Neurosci Lett (1997) 235(1-2):25–8. 10.1016/s0304-3940(97)00706-4
48.
Khan N Lambert-Messerlian G Monteiro JF Hodosy J Tóthová Ľ Celec P et al Oxidative and Carbonyl Stress in Pregnant Women With Obstructive Sleep Apnea. Sleep Breath (2018) 22(1):233–40. 10.1007/s11325-017-1475-8
49.
LeBel CP Bondy SC . Oxygen Radicals: Common Mediators of Neurotoxicity. Neurotoxicol Teratol (1991) 13(3):341–6. 10.1016/0892-0362(91)90081-7
50.
Ng F Berk M Dean O Bush AI . Oxidative Stress in Psychiatric Disorders: Evidence Base and Therapeutic Implications. Int J Neuropsychopharmacol (2008) 11(6):851–76. 10.1017/S1461145707008401
51.
Cumurcu BE Ozyurt H Etikan I Demir S Karlidag R . Total Antioxidant Capacity and Total Oxidant Status in Patients With Major Depression: Impact of Antidepressant Treatment. Psychiatry Clin Neurosci (2009) 63(5):639–45. 10.1111/j.1440-1819.2009.02004.x
52.
Black CN Bot M Scheffer PG Cuijpers P Penninx BW . Is Depression Associated With Increased Oxidative Stress? A Systematic Review and Meta-Analysis. Psychoneuroendocrinology (2015) 51:164–75. 10.1016/j.psyneuen.2014.09.025
53.
Awad SM Morsy H Sayed AA Mohamed NA Ezzat GM Noaman MM . Oxidative Stress and Psychiatric Morbidity in Patients With Facial Acne. J Cosmet Dermatol (2018) 17(2):203–8. 10.1111/jocd.12366
54.
Karababa F Yesilova Y Turan E Selek S Altun H Selek S . Impact of Depressive Symptoms on Oxidative Stress in Patients With Psoriasis. Redox Rep (2013) 18(2):51–5. 10.1179/1351000213Y.0000000039
55.
Steenkamp LR Hough CM Reus VI Jain FA Epel ES James SJ et al Severity of Anxiety-But Not Depression-Is Associated With Oxidative Stress in Major Depressive Disorder. J affective Disord (2017) 219:193–200. 10.1016/j.jad.2017.04.042
56.
Abshirini M Siassi F Koohdani F Qorbani M Mozaffari H Aslani Z et al Dietary Total Antioxidant Capacity Is Inversely Associated With Depression, Anxiety and Some Oxidative Stress Biomarkers in Postmenopausal Women: A Cross-Sectional Study. Ann Gen Psychiatry (2019) 18:3. 10.1186/s12991-019-0225-7
57.
Maes M Bonifacio KL Morelli NR Vargas HO Moreira EG St Stoyanov D et al Generalized Anxiety Disorder (GAD) and Comorbid Major Depression With GAD are Characterized by Enhanced Nitro-Oxidative Stress, Increased Lipid Peroxidation, and Lowered Lipid-Associated Antioxidant Defenses. Neurotox Res (2018) 34(3):489–510. 10.1007/s12640-018-9906-2
58.
Marikovsky M Ziv V Nevo N Harris-Cerruti C Mahler O . Cu/Zn Superoxide Dismutase Plays Important Role in Immune Response. J Immunol (2003) 170(6):2993–3001. 10.4049/jimmunol.170.6.2993
59.
Milaneschi Y Bandinelli S Penninx BW Corsi AM Lauretani F Vazzana R et al The Relationship Between Plasma Carotenoids and Depressive Symptoms in Older Persons. World J Biol Psychiatry (2012) 13(8):588–98. 10.3109/15622975.2011.597876
Summary
Keywords
anxiety, depression, cross-sectional study, sleep, PAB
Citation
Darroudi S, Eslamiyeh M, Jaber Al-Fayyadh KK, Zamiri Bidary M, Danesteh S, Hassanzadeh Gouji A, Darban RA, Esmaily H, Ghayour-Mobarhan M, Moohebati M and Ferns GA (2023) Prognostic Factors Associated With Sleep Duration: Serum Pro-Oxidant/Antioxidant Balance and Superoxide Dismutase 1 as Oxidative Stress Markers and Anxiety/Depression. Int J Public Health 68:1606014. doi: 10.3389/ijph.2023.1606014
Received
22 March 2023
Accepted
14 August 2023
Published
07 September 2023
Volume
68 - 2023
Edited by
Gabriel Gulis, University of Southern Denmark, Denmark
Reviewed by
Yasin Tülüce, Yüzüncü Yıl University, Türkiye
Mustafa Saygin, Süleyman Demirel University, Türkiye
Updates
Copyright
© 2023 Darroudi, Eslamiyeh, Jaber Al-Fayyadh, Zamiri Bidary, Danesteh, Hassanzadeh Gouji, Darban, Esmaily, Ghayour-Mobarhan, Moohebati and Ferns.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohsen Moohebati, mouhebatim@mums.ac.ir
This Original Article is part of the IJPH Special Issue “Public Health and Primary Care, is 1+1=1?”
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.