- 1International School of Public Health and One Health, Hainan Medical University, Haikou, Hainan, China
- 2Emergency Medicine Center, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 3Emergency Department, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
- 4Key Laboratory of Emergency and Trauma of Ministry of Education, Hainan Medical University, Haikou, China
- 5Department of Emergency, Hainan Clinical Research Center for Acute and Critical Diseases, The Second Affiliated Hospital of Hainan Medical University, Haikou, Hainan, China
- 6Research Unit of Island Emergency Medicine, Chinese Academy of Medical Sciences (No. 2019RU013), Hainan Medical University, Haikou, China
Objective: Observational epidemiological studies have shown a link between obesity and sepsis, but any causal relationship is not clear. Our study aimed to explore the correlation and causal relationship between body mass index and sepsis by a two-sample Mendelian randomization (MR).
Methods: In large sample genome-wide association studies, single-nucleotide polymorphisms related to body mass index were screened as instrumental variables. Three MR methods, MR-Egger regression, weighted median estimator, and inverse variance-weighted, were used to evaluate the causal relationship between body mass index and sepsis. Odds ratio (OR) and 95% confidence interval (CI) were used as the evaluation index of causality, and sensitivity analyses were conducted to assess pleiotropy and instrument validity.
Results: By two-sample MR, the inverse variance weighting method results suggested that increased body mass index was associated with an increased risk of sepsis (odds ratio 1.32; 95% CI 1.21–1.44; p = 1.37 × 10−9) and streptococcal septicemia (OR 1.46; 95% CI 1.11–1.91; p = 0.007), but there was no causal relationship with puerperal sepsis (OR, 1.06; 95% CI, 0.87–1.28; p = 0.577). Sensitivity analysis was consistent with the results, and there was no heterogeneity and level of pleiotropy.
Conclusion: Our study supports a causal relationship between body mass index and sepsis. Proper control of body mass index may prevent sepsis.
Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection (1). A systematic review inferred that there are about 30 million sepsis episodes and 6 million deaths worldwide every year (2). Sepsis often leads to the functional involvement of multiple organs in the whole body and induces various complications. Its clinical severity and outcome depend on the location and type of infection, individual response to inflammation, and treatment measures (3). Therefore, improving the prevention, diagnosis, and management of sepsis is of great significance in reducing the global disease burden.
Body mass index (BMI) is an index of the degree of obesity and health. In recent years, it has often been used to predict the risk of related diseases. Observational studies have found an association between BMI and sepsis. High BMI has a protective effect on the mortality of sepsis, but morbid obesity and underweight have nothing to do with the reduction of mortality (4). Due to the influence of confounding factors between exposure and outcome, the causal relationship between BMI and sepsis needs to be further explored.
Mendelian randomization (MR) detects and quantifies the causal relationship between exposure and disease by using genetic variation as an instrumental variable (IV) (5). With the publication of a large amount of exposure and disease-related genetic variation data in genome-wide association studies (GWAS) (6), MR research has made a breakthrough. Compared with observational studies, MR studies using genetic variation as a tool can control for unmeasured confounders and reverse causality (7), which are now mostly used for causal inference of etiology.
Methods
Study Design
We designed a two-sample Mendelian randomization (2SMR) study to explore the causal association between BMI and sepsis (Figure 1). Firstly, the complete summary data set was obtained from the Genetic Investigation of Anthropologic Traits (GIANT) alliance and IEU OpenGWAS open-source database, and the single-nucleotide polymorphisms (SNPs) directly related to BMI were selected as the IV. Secondly, three MR methods, inverse variance-weighted (IVW), weighted median estimator (WME), and MR-Egger regression, were used to analyze the causal relationship. Finally, sensitivity analysis was performed. Cochran’s Q-text was used to evaluate the heterogeneity of MR results, and the level of pleiotropy was determined by MR-Egger regression.
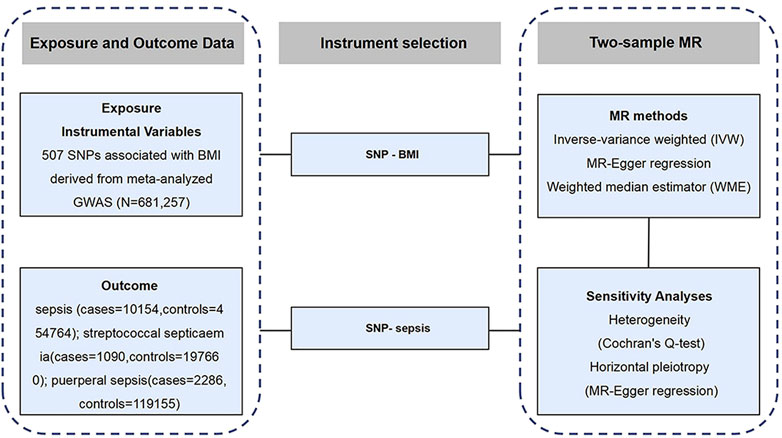
FIGURE 1. Schematic representation of the study design (Exploring the Causality Between Body Mass Index and Sepsis: A Two-Sample Mendelian Randomization Study, China, 2023).
Source and Acquisition of GWAS
The data on BMI were extracted from the meta-analysis of a large GWAS. The database is based on the public database of GIANT alliance. The GWAS results related to BMI in the database were published on human molecular genetics in 2018 (8). The sample size of the study was 681,257 people, and 2,336,260 SNP sites related to BMI were analyzed. The data download website is https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files.
The GWAS data of sepsis came from the IEU OpenGWAS database. A total of 10,154 cases and 454,764 controls were included in the study, all of European descent, consistent with the exposure data. The website for data download is https://gwas.mrcieu.ac.uk/. In addition, GWAS data for streptococcal septicemia and puerperal sepsis were obtained from the FinnGen cohort, which included 96,499 participants and was available from the MR-Base website http://www.mrbase.org/ (9). Sepsis is defined according to the third international consensus on sepsis and septic shock published by the American Medical Association in 2016 (1). All GWAS data on the included studies are available in Supplementary Table S1.
IVs
The SNPs associated with BMI were screened as IVs: (1) SNPs has the significance of genome-wide study (p < 5 × 10−8); (2) The SNPs were kept independent of each other, and the SNPs in linkage disequilibrium were excluded, with R2 > 0.001 and distance located 10,000 kb apart from each other. The MR had three requirements (Figure 2):
(1) IVs have a strong correlation with exposure factors. If the correlation between IVs and exposure is weak, it is easy to produce weak IV bias. It is generally believed that there is no weak IV bias when F > 10, where
(2) IVs are not associated with confounding factors. Genotype should not be associated with confounders in MR—this hypothesis is often difficult to directly prove due to the lack of individual level data. The validity of associations between IV and confounders is sometimes demonstrated using long-accepted criteria in observational epidemiology (11).
(3) IVs have no direct correlation with outcome variables, and can only be related to outcomes through exposure.
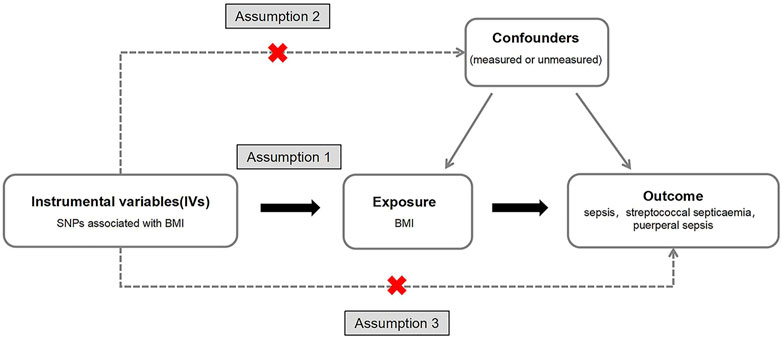
FIGURE 2. A directed acyclic graph of Mendelian random research design. We selected body mass index related single nucleotide polymorphisms as a genetic tool to assess whether body mass index has a causal effect on sepsis. In order to make a causal inference about the impact of body mass index on sepsis, we must also assume that the selected single nucleotide polymorphisms are not related to confounders or outcomes (Exploring the Causality Between Body Mass Index and Sepsis: A Two-Sample Mendelian Randomization Study, China, 2023).
Pleiotropy occurs when a genetic locus affects multiple traits (12). Horizontal pleiotropy can distort the causal estimation of MR and increase the type I error rate (false positive) (13).
Statistical Analysis
In our MR study, IVW was the main statistical method used to test the causal association between BMI and sepsis risk. Results were expressed as the odds ratio (OR) and 95% confidence interval (CI) for the risk of sepsis for each 1 SD increase in BMI. In addition, sensitivity analysis was used to verify the robustness of MR results, including MR-Egger regression to evaluate the bias caused by invalid IVs and pleiotropic IVs, whose intercept term was 0 (p < 0.05), indicating that there was no gene pleiotropy (14). When the effective IVs exceed 50%, the WME method can obtain consistent causal effect estimation (15). Heterogeneity between IVs was assessed using the Cochranʼs Q statistic, with p < 0.05 indicating heterogeneity, which could be controlled by the random-effects IVW.
The above statistical procedures were performed in R software (version 4.1.2) using TwoSampleMR (16) software packages. In addition, the MR-Base platform was used to draw Leave-one-out, scatter, and forest plots of BMI and sepsis-related SNPs to visualize the statistical analysis results, indicating the effectiveness of MR analysis. The test level was a = 0.05, and p < 0.05 was considered significant.
Results
IV Selection for BMI
Details of the SNPS associated with BMI are shown in Supplementary Table S2. After screening, 507 SNPs closely related to BIM were identified as IVs. After reconciling exposure and outcome, 13 palindromic alleles with intermediate frequencies were removed for 2SMR. All SNPs obtained were strong IVs, and F values (range 28–1,426) were all greater than the recommended threshold of 10, indicating that there was no bias caused by weak IVs in the study. Online tools (https://shiny.cnsgenomics.com/mRnd/) were used to calculate each SNPs’ MR analysis of statistical power of sepsis (17).
Mendelian Randomization Between BMI and Sepsis
The IVW method was used for causal analysis, and the results supported the association of genetically predicted BMI with increased risk of sepsis (OR = 1.32, 95% CI = 1.21–1.45, p = 1.37 × 10−9). In sensitivity analysis, Cochran’s Q-test showed no heterogeneity (Cochran’s Q Statistic = 526.30, p = 0.145), and MR-Egger regression analysis showed no pleiotropy (intercept = −0.001; p = 0.474). The causal associations using five different methodological methods (IVW, MR-Egger, Simple Mode, WME, and Weighted Mode) are shown in Table 1. Forest plots showed the causal effect of BMI on sepsis risk (Figure 3). The scatter plot illustrates the causal relationship between BMI and sepsis risk (Figure 4). The leave-one-out plots illustrated that no single SNPs influenced the estimate of the outcome (Supplementary Figure S1).
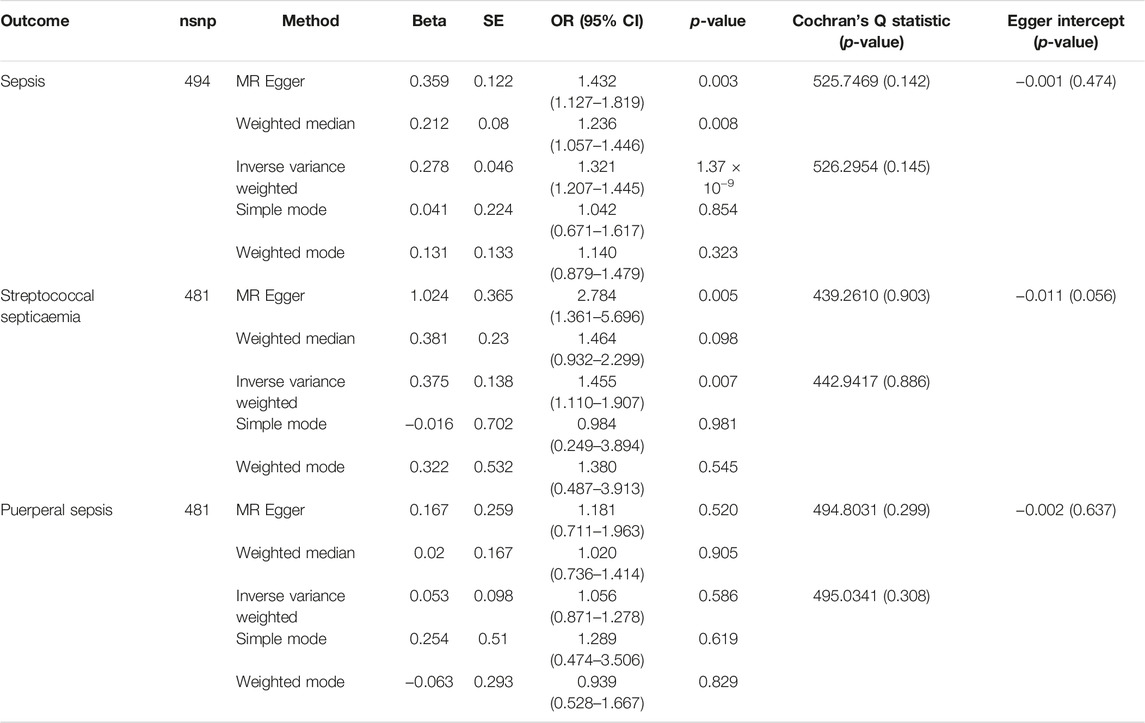
TABLE 1. Mendelian randomization results of the causal effect of body mass index on the risk of sepsis (Exploring the Causality Between Body Mass Index and Sepsis: A Two-Sample Mendelian Randomization Study, China, 2023).
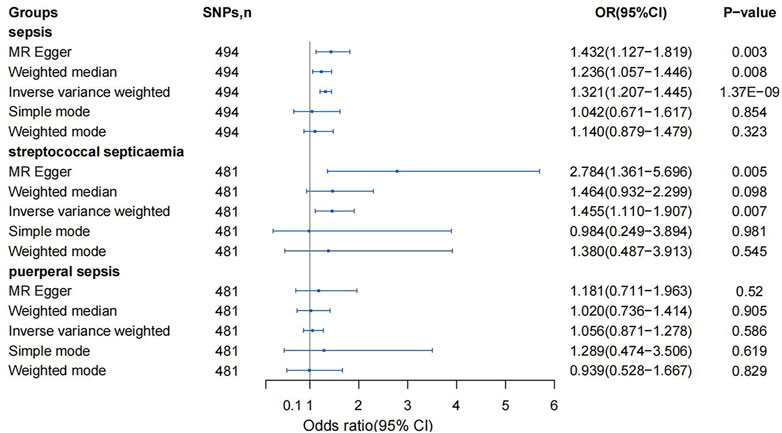
FIGURE 3. Mendelian randomization analysis on the association between body mass index and sepsis (Exploring the Causality Between Body Mass Index and Sepsis: A Two-Sample Mendelian Randomization Study, China, 2023).
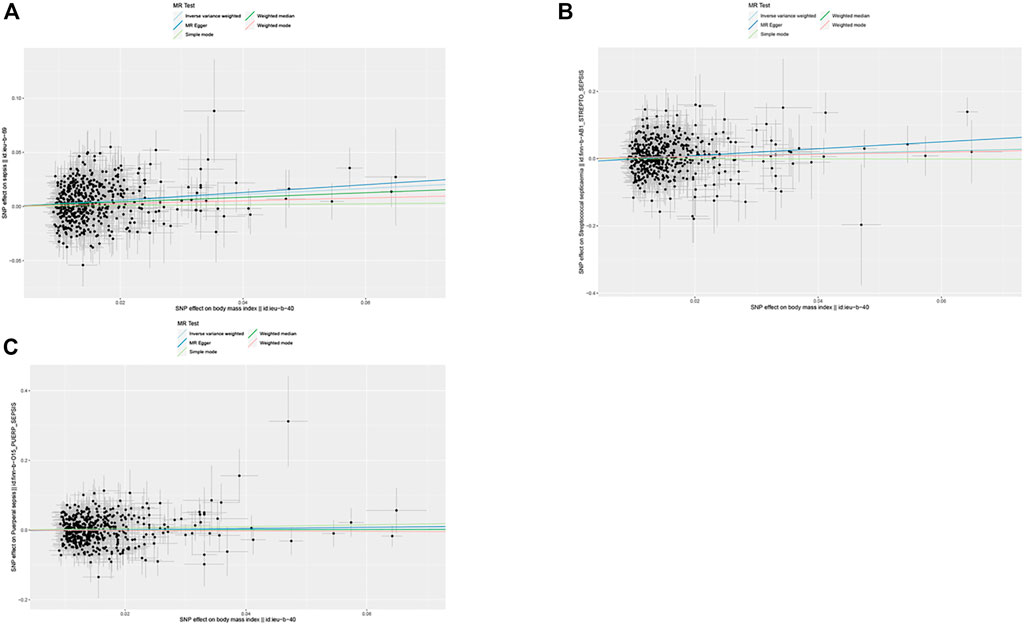
FIGURE 4. Scatter plots of single nucleotide polymorphism effect on body mass index and single nucleotide polymorphism effect on sepsis. (A) Sepsis, (B) streptococcal septicemia, (C) puerperal sepsis (Exploring the Causality Between Body Mass Index and Sepsis: A Two-Sample Mendelian Randomization Study, China, 2023).
MR Between BMI and Streptococcal Sepsis
The MR analysis was performed using 481 SNPs. The IVW results showed a causal relationship between BMI and streptococcal sepsis (OR = 1.46, 95% CI = 1.11–1.91, p = 0.007), without significant heterogeneity (Cochran’s Q Statistic = 442.94, p = 0.886). MR-Egger regression analysis showed no significant difference between the intercept and 0, and there was no pleiotropy (intercept = −0.011; p = 0.056).
MR Between BMI and Puerperal Sepsis
The 2SMR results showed no causal relationship between BMI and postpartum sepsis risk (IVW, OR = 1.06, 95% CI = 0.87–1.28, p = 0.577; MR-Egger, OR = 1.18, 95% CI = 0.71–1.96, p = 0.523), heterogeneity (Cochran’s Q Statistic = 493.56, p = 0.325) and pleiotropy (intercept = −0.002; p = 0.645).
Discussion
We designed a 2SMR study to assess whether human genetic evidence supports a causal association between BMI and sepsis (or sepsis). The results showed that elevated BMI was associated with an increased risk of sepsis and streptococcal sepsis, but not postpartum sepsis. This finding was consistent across different analytical methods, and the association between BMI and sepsis was considered robust when combined with a series of sensitivity analyses.
Sepsis is a common and frequently occurring disease in hospital emergency departments, and is one of the major problems affecting human health. High BMI may induce sepsis through some pathophysiological mechanism (18). Early studies suggested that obesity can induce production of proinflammatory cytokines such as tumor necrosis factor (TNF-a) in adipose tissue, leading to inflammatory response in patients with sepsis (19). These inflammatory processes involve the upregulation of systemic immunity, which is clinically manifest as insulin resistance and metabolic syndrome, increasing the risk of organ failure, infectious disease and death in patients (20). The second possible explanation is that the process of oxidative stress is significantly worsened in obese patients with sepsis (21). An animal experiment showed that obese animals were more likely to suffer from the harmful effects of sepsis on peripheral organs due to oxidative damage and low antioxidant levels of enzymes (22).
Observational epidemiological studies have confirmed the association between BMI and sepsis. In a retrospective cohort analysis of 834 patients, among septic patients admitted to the ICU, obese patients were associated with higher mortality than nonobese patients (23). Due to the dysfunction of the immune system in obese people, the prevalence and mortality of sepsis are significantly increased (24). However, this association is not robust, and some studies suggest the exact opposite. Mica et al. believe that high BMI has a protective effect on sepsis (25), which may be related to the increase of leptin in obese people (26). Confusion in causal inferences in observational studies can lead to completely opposite conclusions. Therefore, in this study, we evaluated the causal effect estimates between exposure factors and outcome variables by MR studies and using genetic IV.
Although studies have shown some association between BMI and sepsis, correlation is not equal to causality, and exploring relevant but non-causal influencing factors is not ideal for the prevention and treatment of sepsis. Therefore, understanding the genetic role of sepsis is the key to explaining its pathogenesis and proposing preventive strategies. A recently published MR study associated increased BMI with increased sepsis mortality at 28 days, although the effect disappeared at 90 days (27). In another MR study on BMI and infection risk, high BMI was associated with an increased risk of sepsis (28). Our results supported these two previous studies.
In addition, our study showed that high BMI increased the risk of streptococcal sepsis. Obesity is a risk factor for increased mortality in patients with bacteremia, and bacteremia is one cause of sepsis (29). To our knowledge, this is the first MR study to investigate the association between BMI and the risk of postpartum sepsis. Retrospective observational studies have found that after controlling for the mode of delivery, obese women are twice as likely as normal-weight women to develop the complication of sepsis (30). However, our study showed no causal link, which may be due to the increased incidence and mortality of sepsis during puerperium due to maternal infection with streptococci (30).
We used large samples of GWAS data and detailed validation of MR hypotheses to improve the efficiency of MR analysis. The GWAS data on exposure and outcomes were obtained from individuals of European ancestry, reducing the results of the study by factors such as population stratification. We used several analytical methods to assess the possibility of pleiotropy and showed that the results of WME and MR-Egger regression were similar to those of IVW. More IVs, more power, more statistical methods, and heterogeneity-testing methods can improve the robustness of MR results and prevent reverse causality bias.
There are some limitations to our study. First, when we investigated whether there was a causal relationship between obesity and sepsis, we used BMI, which represents total body obesity, rather than the waist circumference metric used to assess centripetal obesity, despite observational studies concluding that abdominal obesity is also associated with an increased risk of death from sepsis (21). Second, while statistically significant, our study had only a 32% increase in the risk of sepsis for a genetically induced one-unit increase in BMI. Third, because BMI is a highly polygenic trait, pleiotropy cannot be entirely ruled out. Some studies have observed a positive correlation between BMI and inflammatory biomarkers, and excessive inflammation is thought to be the root cause of early organ dysfunction in sepsis (31). As a result, more research is needed to determine whether inflammatory markers mediate the causal relationship between BMI and sepsis. Fourth, our study design was not stratified for BMI, and there is still a lack of data for a more detailed assessment of the “obesity paradox” (20) that may arise from extreme BMI. Fifth, the GWAS included were from European populations. Therefore, considering the differences in body size between European, North American, and Asian people, there may be limitations in the generalization of the results to other populations.
Conclusion
In conclusion, high BMI was associated with an increased risk of sepsis and streptococcal sepsis, independent of the occurrence of postpartum sepsis. In contrast to observational studies, MR analyses used genetic IVs to estimate the effect of exposure on disease incidence, and our findings further support the finding that appropriate management of BMI may prevent the development of sepsis, a finding with important public health implications.
Author Contributions
JW: Software, Formal analysis, Data Curation, Writing—Original Draft. YH: Validation, Data Curation, Writing—Original Draft. QL: Methodology, Data Curation, Writing—Review and Editing. JZ: Project administration. LH: Validation, Writing—Review and Editing. WH: Validation, Writing—Review and Editing. XS: Data Curation. SY: Conceptualization, Writing—Review and Editing, Project administration. CL: Conceptualization, Writing—Review and Editing, Supervision.
Funding
This study was supported by Hainan Province Science and Technology Special Fund (ZDKJ202004, ZDKJ2021038), Hainan Provincial Natural Science Foundation of China (821RC557, 2019RC232), National Natural Science Foundation of China (81871611, 82160647), Finance Science and Technology Program of Sichuan Province (2022YFS0602).
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Acknowledgments
The authors want to acknowledge all the participants and investigators of the GWASs involved in the present study for generously sharing the summary-level data.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2023.1605548/full#supplementary-material
Supplementary Table S1 | Descriptive characteristics of the GWAS meta-analyses used in the current Mendelian randomization study.
Supplementary Table S2 | Characteristics of the genetic variants associated with sepsis.
Supplementary Figure S1 | Leave-one-out plot. (A) Sepsis, (B) streptococcal septicemia, (C) puerperal sepsis.
Abbreviations
BMI, Body mass index; MR, Mendelian randomization; GWAS, Genomic-wide association study; SNP, Single-nucleotide polymorphism; IV, Instrumental variable; 2SMR, Two-sample Mendelian randomization; IVW, Inverse variance-weighted; MR-Egger, Mendelian randomization-Egger regression; WME, Weighted median estimator; OR, Odds ratio; SD, Standard deviation; TNF-α, Tumor necrosis factor-alpha.
References
1. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA (2016) 315:801–10. doi:10.1001/jama.2016.0287
2. Fleischmann, C, Scherag, A, Adhikari, NKJ, Hartog, CS, Tsaganos, T, Schlattmann, P, et al. Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med (2016) 193:259–72. doi:10.1164/rccm.201504-0781OC
3. Cave, MC, Hurt, RT, Frazier, TH, Matheson, PJ, Garrison, RN, McClain, CJ, et al. Obesity, Inflammation, and the Potential Application of Pharmaconutrition. Nutr Clin Pract : official Publ Am Soc Parenter Enteral Nutr (2008) 23:16–34. doi:10.1177/011542650802300116
4. Cichon, I, Ortmann, W, Santocki, M, Opydo-Chanek, M, and Kolaczkowska, E. Scrutinizing Mechanisms of the 'Obesity Paradox in Sepsis': Obesity Is Accompanied by Diminished Formation of Neutrophil Extracellular Traps (NETs) Due to Restricted Neutrophil-Platelet Interactions. CELLS (2021) 10:384. doi:10.3390/cells10020384
5. Palmer, TM, Lawlor, DA, Harbord, RM, Sheehan, NA, Tobias, JH, Timpson, NJ, et al. Using Multiple Genetic Variants as Instrumental Variables for Modifiable Risk Factors. Stat Methods Med Res (2012) 21:223–42. doi:10.1177/0962280210394459
6. Visscher, PM, Wray, NR, Zhang, Q, Sklar, P, McCarthy, MI, Brown, MA, et al. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am J Hum Genet (2017) 101:5–22. doi:10.1016/j.ajhg.2017.06.005
7. Davey Smith, G, and Hemani, G. Mendelian Randomization: Genetic Anchors for Causal Inference in Epidemiological Studies. Hum Mol Genet (2014) 23:R89–R98. doi:10.1093/hmg/ddu328
8. Yengo, L, Sidorenko, J, Kemper, KE, Zheng, Z, Wood, AR, Weedon, MN, et al. Meta-analysis of Genome-wide Association Studies for Height and Body Mass index in ∼700000 Individuals of European Ancestry. Hum Mol Genet (2018) 27:3641–9. doi:10.1093/hmg/ddy271
9. Si, S, Li, J, Tewara, MA, and Xue, F. Genetically Determined Chronic Low-Grade Inflammation and Hundreds of Health Outcomes in the UK Biobank and the FinnGen Population: A Phenome-wide Mendelian Randomization Study. Front Immunol (2021) 12:720876. doi:10.3389/fimmu.2021.720876
10. Pierce, BL, and Burgess, S. Efficient Design for Mendelian Randomization Studies: Subsample and 2-Sample Instrumental Variable Estimators. Am J Epidemiol (2013) 178:1177–84. doi:10.1093/aje/kwt084
11. Glymour, MM, Tchetgen, EJT, and Robins, JM. Credible Mendelian Randomization Studies: Approaches for Evaluating the Instrumental Variable Assumptions. Am J Epidemiol (2012) 175:332–9. doi:10.1093/aje/kwr323
12. Verbanck, M, Chen, C-Y, Neale, B, and Do, R. Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred from Mendelian Randomization between Complex Traits and Diseases. Nat Genet (2018) 50:693–8. doi:10.1038/s41588-018-0099-7
13. Fujii, T, Ganeko, R, Kataoka, Y, Furukawa, TA, Featherstone, R, Doi, K, et al. Correction to: Polymyxin B-Immobilized Hemoperfusion and Mortality in Critically Ill Adult Patients with Sepsis/septic Shock: a Systematic Review with Meta-Analysis and Trial Sequential Analysis. INTENSIVE CARE MEDICINE (2018) 44:279–80. doi:10.1007/s00134-018-5055-6
14. Bowden, J, Del Greco, MF, Minelli, C, Davey Smith, G, Sheehan, NA, and Thompson, JR. Assessing the Suitability of Summary Data for Two-Sample Mendelian Randomization Analyses Using MR-Egger Regression: the Role of the I2 Statistic. Int J Epidemiol (2016) 45:1961–74. doi:10.1093/ije/dyw220
15. Bowden, J, Smith, GD, Haycock, PC, and Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol (2016) 40:304–14. doi:10.1002/gepi.21965
16. Hemani, G, Zhengn, J, Elsworth, B, Wade, KH, Haberland, V, Baird, D, et al. The MR-Base Platform Supports Systematic Causal Inference across the Human Phenome. Elife (2018) 7:e34408. doi:10.7554/eLife.34408
17. Brion, M-JA, Shakhbazov, K, and Visscher, PM. Calculating Statistical Power in Mendelian Randomization Studies. Int J Epidemiol (2013) 42:1497–501. doi:10.1093/ije/dyt179
18. Arabi, YM, Dara, SI, Tamim, HM, Rishu, AH, Bouchama, A, Khedr, MK, et al. Clinical Characteristics, Sepsis Interventions and Outcomes in the Obese Patients with Septic Shock: an International Multicenter Cohort Study. Crit Care (2013) 17:R72. doi:10.1186/cc12680
19. Kolyva, AS, Zolota, V, Mpatsoulis, D, Skroubis, G, Solomou, EE, Habeos, IG, et al. The Role of Obesity in the Immune Response during Sepsis. Nutr Diabetes (2014) 4:e137. doi:10.1038/nutd.2014.34
20. Pepper, DJ, Demirkale, CY, Sun, J, Rhee, C, Fram, D, Eichacker, P, et al. Does Obesity Protect against Death in Sepsis? A Retrospective Cohort Study of 55,038 Adult Patients. Crit Care Med (2019) 47:643–50. doi:10.1097/ccm.0000000000003692
21. Wang, HE, Griffin, R, Judd, S, Shapiro, NI, and Safford, MM. Obesity and Risk of Sepsis: A Population-Based Cohort Study. Obesity (2013) 21:E762–9. doi:10.1002/oby.20468
22. Petronilho, F, Della Giustina, A, Nascimento, DZ, Zarbato, GF, Vieira, AA, Florentino, D, et al. Obesity Exacerbates Sepsis-Induced Oxidative Damage in Organs. Inflammation (2016) 39:2062–71. doi:10.1007/s10753-016-0444-x
23. Papadimitriou-Olivgeris, M, Aretha, D, Zotou, A, Koutsileou, K, Zbouki, A, Lefkaditi, A, et al. The Role of Obesity in Sepsis Outcome Among Critically Ill Patients: A Retrospective Cohort Analysis. Biomed Res Int (2016) 2016:5941279. doi:10.1155/2016/5941279
24. Frydrych, L, Bian, G, O'Lone, DE, Ward, PA, and Delano, MJ. Obesity and Type 2 Diabetes Mellitus Drive Immune Dysfunction, Infection Development, and Sepsis Mortality. J Leukoc Biol (2018) 104:525–34. doi:10.1002/JLB.5VMR0118-021RR
25. Mica, L, Vomela, J, Keel, M, and Trentz, O. The Impact of Body Mass index on the Development of Systemic Inflammatory Response Syndrome and Sepsis in Patients with Polytrauma. Injury (2014) 45:253–8. doi:10.1016/j.injury.2012.11.015
26. Wang, S, Liu, X, Chen, Q, Liu, C, Huang, C, and Fang, X. The Role of Increased Body Mass index in Outcomes of Sepsis: a Systematic Review and Meta-Analysis. BMC Anesthesiol (2017) 17:118. doi:10.1186/s12871-017-0405-4
27. Butler-Laporte, G, Harroud, A, Forgetta, V, and Richards, JB. Elevated Body Mass index Is Associated with an Increased Risk of Infectious Disease Admissions and Mortality: a Mendelian Randomization Study. Clin Microbiol Infect (2021) 27:710–6. doi:10.1016/j.cmi.2020.06.014
28. Winter-Jensen, M, Afzal, S, Jess, T, Nordestgaard, BG, and Allin, KH. Body Mass index and Risk of Infections: a Mendelian Randomization Study of 101,447 Individuals. Eur J Epidemiol (2020) 35:347–54. doi:10.1007/s10654-020-00630-7
29. Reetta, H, Janne, L, Jukka, L, Risto, V, and Jaana, S. Obesity and Smoking Are Factors Associated with Poor Prognosis in Patients with Bacteraemia. BMC Infect Dis (2007) 7:13. doi:10.1186/1471-2334-7-13
30. Acosta, CD, Bhattacharya, S, Tuffnell, D, Kurinczuk, JJ, and Knight, M. Maternal Sepsis: a Scottish Population-Based Case-Control Study. BJOG : Int J Obstet Gynaecol (2012) 119:474–83. doi:10.1111/j.1471-0528.2011.03239.x
Keywords: obesity, body mass index, Mendelian randomization, sepsis, instrumental variable
Citation: Wang J, Hu Y, Zeng J, Li Q, He L, Hao W, Song X, Yan S and Lv C (2023) Exploring the Causality Between Body Mass Index and Sepsis: A Two-Sample Mendelian Randomization Study. Int J Public Health 68:1605548. doi: 10.3389/ijph.2023.1605548
Received: 31 October 2022; Accepted: 19 April 2023;
Published: 02 May 2023.
Edited by:
Gabriel Gulis, University of Southern Denmark, DenmarkReviewed by:
Fatme Al Anouti, Zayed University, United Arab EmiratesCopyright © 2023 Wang, Hu, Zeng, Li, He, Hao, Song, Yan and Lv. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shijiao Yan, eWFuc2hpamlhb0BoYWlubWMuZWR1LmNu; Chuanzhu Lv, THZjaHVhbnpodTY3N0AxMjYuY29t
†These authors have contributed equally to this work
This Original Article is part of the IJPH Special Issue “Public Health and Primary Care, is 1+1=1?“
 Juntao Wang
Juntao Wang Yanlan Hu1†
Yanlan Hu1†