- 1Postgraduate Program in Health Sciences, Federal University of Goiás, Goiânia, Brazil
- 2Advanced Center for Breast Diagnosis (CORA), Federal University of Goiás, Goiânia, Brazil
- 3Goiás State Education Department, Goiânia, Brazil
Objectives: This study aimed to evaluate COVID-19 effects on breast cancer screening and clinical stage at diagnosis in patients of 50–69 years of age receiving care within the public healthcare network (SUS) in 2013–2021 in Brazil and its macro-regions.
Methods: This ecological study used Poisson regression to analyze trends in screening and staging. A secondary database was formed using SUS sources: outpatient data system of the SUS network and Oncology—Brazil Panel.
Results: There was a reduction in screening, with an annual percent change of −5.9 (p < 0.022). The number of notified cases fell by 31.5% in 2020–2021 compared to 2018–2019. There was a 10.7% increase in the proportion of stage III/IV cases (p < 0.001) in 2020–2021 compared to 2013–2019, now surpassing the number of cases of early stage breast cancer.
Conclusion: COVID-19 led to a reduction in breast cancer screening and an expressive increase in advanced tumors in users of the public healthcare network. Urgent interventions in public policies are required as the negative effects of the pandemic on the diagnosis/treatment of breast cancer are becoming apparent even earlier than expected.
Introduction
The COVID-19 pandemic impacted healthcare services worldwide due to the need to prioritize resources for urgencies and reduce dissemination of the virus at healthcare services (1). Consequently, there were negative repercussions on elective procedures, including cancer screening (2–4), which was paused in Brazil and in the vast majority of countries (2, 5, 6).
According to data published by the World Health Organization, over half the countries investigated interrupted treatment services for hypertension either partially or totally, while 49% interrupted the treatment of diabetes and its complications; 42% interrupted cancer treatment; and 31% suspended cardiovascular emergencies. Furthermore, 50% of these countries reported a reduction in public screening programs (7). National and international medical societies published recommendations on cancer care, taking individual risks and benefits to patients into consideration (4, 8–11).
As the pandemic progressed and vaccination programs were implemented, the restrictive measures hitherto imposed were eased somewhat, allowing healthcare managers and professionals to evaluate whether to begin offering elective procedures, particularly the screening, diagnosis and early treatment of malignant neoplasms (3, 12). Deciding on these issues was of utmost importance in view of the real risk that the delay in cancer treatment during the pandemic would increase cancer-related morbidity and mortality (13).
In addition to the set of restrictive measures implemented as public policies to combat the pandemic, many women opted to delay healthcare treatment because they were worried about COVID-19 infection and its complications (14).
In this respect, breast cancer was particularly concerning, as it is the most common form of cancer, with an estimated 2.3 million new cases diagnosed annually worldwide (15). Many medical associations voiced their concerns regarding the impact of the restrictive measures on breast cancer (9, 10, 16). Indeed, studies published in the literature have already shown a decrease in the number of breast cancer diagnoses and in breast cancer screening, with no sign of a return to pre-pandemic levels (17–20). However, the eventual repercussions of this difficulty in providing appropriate care were yet to be ascertained, particularly with respect to diagnosing cases at more advanced stages of the disease (18).
With the pandemic still ongoing and the general lack of data on the subject, the objective of the present study was to evaluate the possible decrease in breast cancer screening and the increase in the percentage of advanced clinical stage in women aged 50–69 years, compared with previous years, in Brazil. This age group constitutes the brazilian target population recommended in the Guidelines for the Early Detection of Breast Cancer (21).
Methods
This was an ecological study designed to evaluate temporal changes in which data on breast cancer screening and on the clinical stages of breast cancer were analyzed for the period from 2013 to 2021 with respect to women of 50–69 years of age receiving care within the public healthcare system. Data were analyzed for Brazil as a whole and for its five geographical regions (the north, northeast, southeast, south and mid-west).
A secondary database was formed using various different sources: data on the number of mammograms performed were extracted from the outpatient data system of the SUS network (SIA/DATASUS) (22), while the data on clinical staging at diagnosis were obtained from the SUS’s Oncology—Brazil Panel (DATASUS) (23). In addition, the data used to calculate the target population in the country were acquired from the Brazilian Institute of Geography and Statistics (IBGE) (24), and the data used to calculate the size of the female population covered by supplementary healthcare were obtained from the National Agency for Supplementary Healthcare (ANS) (25).
The internal review board of the Teaching Hospital, Federal University of Goiás approved the study protocol under reference CAAE 56747022.0.0000.5078. Since the study was conducted using freely available, unrestricted secondary data, the requirement for signed informed consent was waived.
Target Population
The intercensal projected population of Brazil established by the “Projections of the population of Brazil and Federation Units by sex and age: 2010–2060” (24), updated in 2018, was used for the 2013–2021 period. From this population projection, the percentage of women who had private health insurance was extracted for each year of the period studied (data available at ANS).
Breast Cancer Screening Coverage
The number of exams carried out annually between 2013 and 2021 was obtained from the DATASUS outpatient database (22). Two procedure codes were taken into consideration: bilateral screening mammogram (02.04.03.018-8) and mammogram (02.04.03.003-0 40). As far as data on breast cancer screening are concerned, underreporting is unlikely, since healthcare institutes need to register the mammograms performed in order to receive payment for services provided.
Accordingly the Technical Parameters for Breast Cancer Screening (26), to provide a more realistic estimate of the number of procedures performed, the breast cancer screening coverage goal to be achieved at the established period took the supplementary healthcare coverage at each location into account so as not to overestimate the procedures available within the public healthcare program.
Therefore, the percentage of women with private health insurance were obtained from the ANS and was subtracted from the target population (25). Based on the information obtained, the expected number of mammograms for the year in question was calculated.
The expected number of exams for the target population was calculated from the total number of women of 50–69 years of age, the percentage of women with private health insurance was subtracted from the target population in accordance with the recommendations of the National Cancer Institute for two-yearly screening (26).
Estimated coverage was calculated based on biannual screening of 100% of the target population. This indicator was expressed as a percentage and calculated from the ratio of the number of mammograms performed and the number of expected mammograms for the target population (26, 27).
Clinical Staging
In order to extract the data on clinical staging from the oncology panel platform (23), the only filter applied was that used to select breast cancer stages I, II, III and IV, referring to the stage registered at chemotherapy, radiotherapy or both. These data were grouped as follows: 1) stages I/II, representing the initial stages of breast cancer, and 2) stages III/IV, representing advanced stages of the disease. Stage 0, which refers to ductal carcinoma in situ, a pre-cancerous manifestation that is not considered breast cancer, was excluded.
Statistical Analysis
Trends in the rates of breast cancer screening were evaluated according to the annual percent change (APC) in the estimated breast cancer screening coverage in Brazil and its regions. The Poisson regression model was applied in the analysis using JoinPoint Regression, version 4.9.0.1 (28). The 95% confidence intervals (95%CI) were calculated, with p-values <0.05 being considered statistically significant.
In the analysis and interpretation of the results, an increase in estimated breast screening coverage was assumed when the APC was positive, and the minimum value of the confidence interval was above zero. Conversely, breast-screening coverage was considered to have decreased when the APC was negative, and the maximum value of the confidence interval was below zero. Coverage was considered to have stabilized when, irrespective of the amount of coverage, the minimum value of the confidence interval was below zero and the maximum value was above zero.
Staging was characterized using absolute and relative frequencies calculated for Brazil as a whole and for the different regions. Comparison of staging prior to the pandemic (2013–2019) and during the pandemic (2020–2021), for Brazil and for the different regions, was performed using McNemar’s test. Poisson regression analysis was used to analyze trends in staging between 2013 and 2021. Coverage in the pre-pandemic period and coverage during the pandemic were compared using the Mann-Whitney U test. The data were analyzed using the Statistical Package for the Social Sciences (SPSS), version 26.0 (29). A 5% significance level was adopted (p < 0.05).
Results
A total of 1,613,119 mammograms were performed within the public healthcare service in Brazil in 2020, a number that is 40% lower than the 2,658,289 mammograms performed in 2019. In 2021, this number was 2,189,734, still 18% lower compared to 2019.
A reduction in breast cancer screening coverage was found for the 2013–2019 period, with an APC of −1.78 (p < 0.040; 95% CI −3.4–−2.8), while for the 2013–2021 period, was found an APC of −5.85 (p < 0.02; 95% CI −10.3–−1.2), as shown in Figure 1. Compared to 2019, coverage decreased by 41% in 2020 and by 21% in 2021 (Table 1).
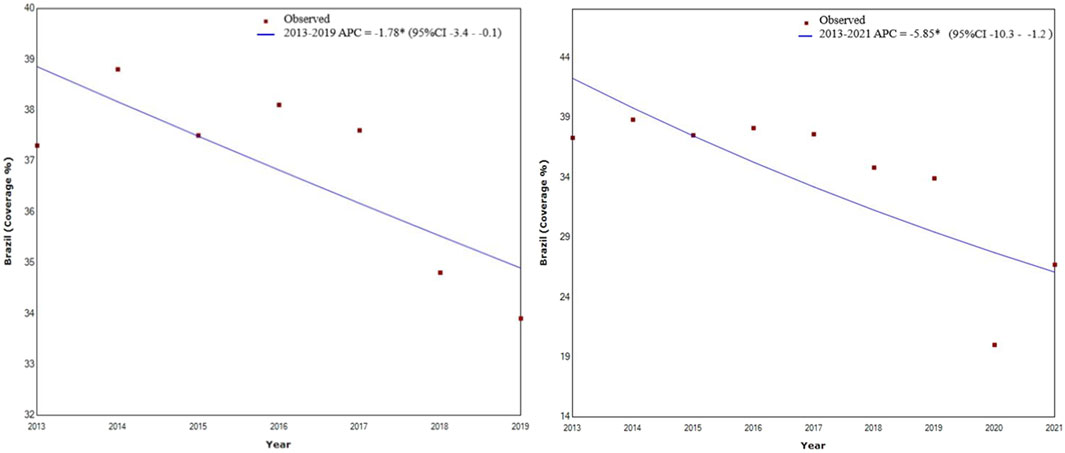
FIGURE 1. Trend in the rate of breast cancer screening coverage within the public healthcare system in Brazil between 2013–2019 and 2013–2021 in women of 50–69 years of age (Poisson regression model). Brazil, 2013–2021. APC, Annual Percentual Change; 95%CI, 95% Confidence Interval.
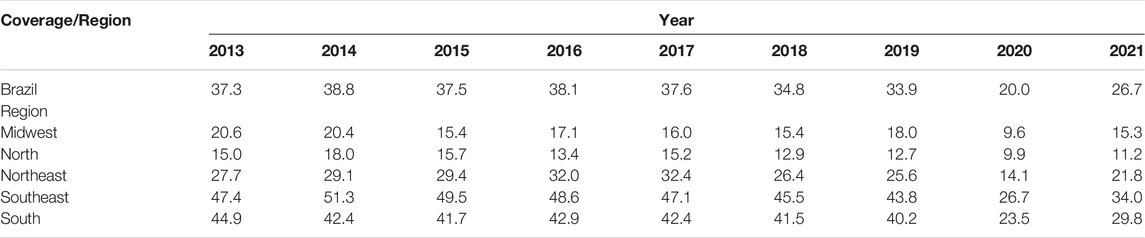
TABLE 1. Percentage rate of breast cancer screening coverage in Brazil as a whole and in its individual regions in women of 50–69 years of age receiving care within the Brazilian public healthcare service (SUS) between 2013 and 2021 (Brazil, 2013–2021).
When the 2013–2019 and 2020–2021 periods were compared, a reduction was found in the mean rate of breast cancer screening coverage in Brazil from 36.71 ± 1.73 to 21.97 ± 3.02 (p < 0.01). A similar reduction was found in all the regions of the country: mid-west: from 17.50 ± 2.01 to 11.15 ± 2.52 (p < 0.001); northeast: from 28.79 ± 2.50 to 16.30 ± 3.49 (p < 0.001); north: from 14.55 ± 1.70 to 10.24 ± 0.57 (p < 0.001), southeast: from 47.41 ± 2.35 to 28.85 ± 3.28 (p < 0.001), and south: from 42.24 ± 1.39 to 25.29 ± 2.87 (p < 0.001), as shown in Table 2.
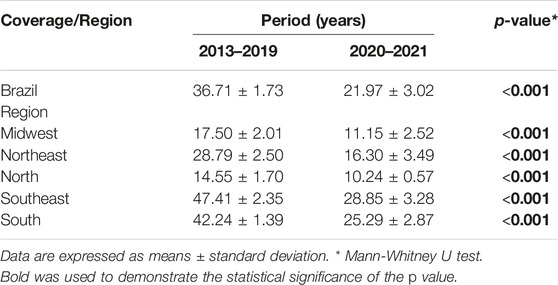
TABLE 2. Comparison of breast cancer screening coverage between 2013–2019 and 2020–2021 (Brazil, 2013–2021).
During the 2018–2019 period, a total of 26,675 cases of breast cancer were registered for the country as a whole compared to 18,284 in 2020–2021, representing a reduction of 31.5% and highlighting the impact of the pandemic on the total number of cases identified.
In parallel, when 2013–2019 was compared to 2020–2021, a 10.7% increase was found in the proportion of cases of stage III/IV breast cancer (p < 0.001), which now surpass the proportion of cases of stage I/II for Brazil as a whole. Analysis of the different regions showed a statistically significant proportional increase in advanced stages, with a 21.3% increase in the mid-west, a 10.3% increase in the northeast, 16.5% in the north, 11.2% in the southeast and 6.2% in the south (Table 3). In all the regions except for the south, the percentage of cases of stage III/IV surpassed that of stage I/II during the pandemic (Table 3).
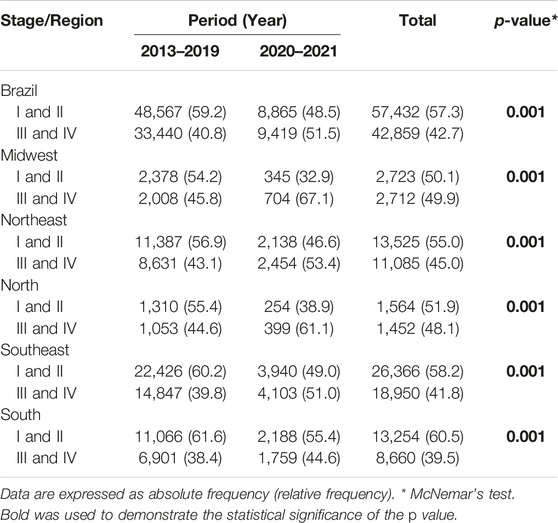
TABLE 3. Breast cancer stages at diagnosis prior to the COVID-19 pandemic (2013–2019) compared to those during the pandemic (2020–2021) for Brazil as a whole and its regions (Brazil, 2013–2021).
According to the Poisson regression analysis, when comparing the coefficient of determination (r2) of the trend in clinical staging of breast cancer from 2013–2019 (r2 = 0.48; 95%CI 38.9–47.9; p < 0.001) with 2013–2021 (r2 = 0.85; 95% CI 38.9–47.9; p < 0.001), there is an increase in staging III and IV in the period that includes the pandemic over the percentage in 2013–2019, as shown in Figure 2.
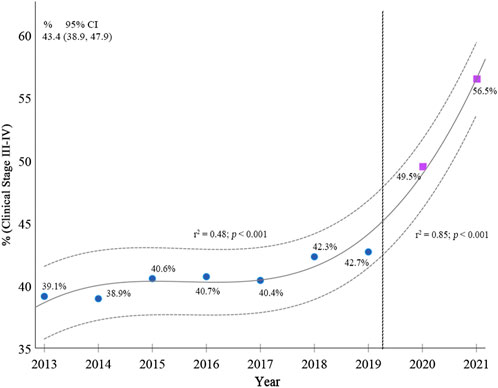
FIGURE 2. Breast cancer stages from 2013 to 2021 in women of 50–69 years of age (Poisson regression model). Brazil, 2013–2021.
Discussion
The results of the present study show that during the pandemic breast cancer screening coverage in women in the target group established by the Ministry of Health decreased in Brazil as a whole and in all its regions. Even after the restrictive measures implemented in the country had eased, breast cancer screening coverage failed to return to pre-pandemic levels. This finding is in line with observations from several countries (18), with a possible impact on the diagnosis of breast cancer, its staging, treatment and associated mortality rates.
In Brazil, cancer screening and diagnosis were more severely affected than treatment, irrespective of the type of cancer (11). A reduction was found in the number of breast cancer diagnoses, with the cases detected suggestive of a poorer prognosis (30), as also shown in our study. The proportion of palpable nodules was significantly greater in 2020 compared to 2019 (31).
A reduction of 40% was found in breast cancer screening in Brazil between 2019 and 2020. Other studies have reported reductions ranging from 35.3% to 42% (11, 31). In 2021, even after the restrictive measures had been eased and vaccine programs implemented, breast cancer screening coverage remained 21% lower compared to the years that preceded the pandemic. This finding is concerning in a country in which breast cancer screening coverage was already poor, as shown in the present study and in previous publications (27, 32, 33).
The inequalities that exist in Brazil were compounded by the effect of the pandemic. As shown, there was a greater reduction in the screening rate in the regions of the country in which the human development index (HDI) is lower such as in the north, northeast and mid-west. This finding is in agreement with various studies conducted in Brazil showing the correlation between breast cancer screening and regional differences in the availability of healthcare services (32, 34, 35).
The present study showed a reduction in the absolute numbers of cases of cancer notified and a proportional increase in the number of cases diagnosed at more advanced stages during the COVID-19 pandemic, with the number of cases of stages III and IV now surpassing that of stages I and II in all the regions of the country except for the south. Studies conducted both in developed and in developing countries (36, 37) have also shown a reduction in the number of cases diagnosed (17, 37) and an increase in the number of diagnoses at more advanced stages (30, 38).
To the best of our knowledge, the present study is the first to evaluate data for Brazil as a whole and for its individual regions, with the results highlighting the pronounced consequences of the pandemic throughout the entire country for patients within the public healthcare network. Prior to the pandemic, 40.8% of cases of breast cancer in women of 50–69 years of age were diagnosed at stages III/IV, with this proportion increasing significantly to 51.5% between 2020 and 2021.
The increase in breast cancer diagnoses at advanced stages in Brazil as a consequence of delayed diagnosis could result in an increase in more mutilating surgeries and a need for adjuvant and neoadjuvant treatment that could have been avoided (1, 39). Other factors to take into consideration are a possible increase in the number of avoidable deaths (40), deterioration in the quality of life of surviving patients and a rise in health-related expenditure (1, 40).
The impact on morbidity and mortality will depend on the duration of the interruption to screening. For this reason, restoring trust and guaranteeing the safety of patients participating in screening programs is crucial (39, 40), as is the implementation of actions aimed at raising awareness and encouraging screening (19).
Interventions in the form of public policies need to be implemented urgently in an attempt to increase screening and patient access, expanding coverage beyond the levels seen prior to the pandemic. These actions are crucial in order to minimize the consequences of delayed diagnosis and treatment of breast cancer patients, bearing in mind that the negative effects of the pandemic are becoming apparent earlier than expected.
Limitations
Since the data were obtained from a secondary source, the analysis was restricted to the data supplied from SIA/DATASUS, the Oncology-Brazil Panel, ANS and IBGE, and this constitutes a limitation of the present study. There is a possibility that cases of cancer registered with the Oncology-Brazil Panel could be underreported. About breast cancer screening, the data are quite reliable because mammograms are paid for only when they are recorded in the system. Therefore, the likelihood of underreporting is minimal.
Conclusion
During the COVID-19 pandemic, there was a significant reduction in the number of mammograms performed within the public healthcare system in Brazil, with a reduction in breast cancer screening coverage together with an expressive increase in the number of patients with advanced tumors (clinical stages III and IV) at presentation. The speed at which this increase in advanced stages of the disease occurred is noteworthy and indicates that breast cancer screening should be reinitiated as quickly as possible in order to avoid the impact of late diagnosis.
Ethics Statement
The studies involving human participants were reviewed and approved by Teaching Hospital, Federal University of Goiás approved the study protocol under reference CAAE 56747022.0.0000.5078. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AR: Conceived and designed the study; collected the data; constructed and analyzed the database; wrote the initial draft of the paper; revised and edited the final manuscript. RdF: Coordinated the study; conceived and designed the study; interpreted the data; edited and critically reviewed the final manuscript. GF: Collected the data and constructed the database, contributed to writing the first draft of the paper and the presentation of the results; reviewed and edited the final version of the manuscript. DR: Contributed to constructing the database and drafting the initial manuscript; reviewed and edited the final version of the paper. RR: Contributed in the data analysis and in writing the initial draft manuscript; edited the final version of the manuscript. All the authors read and approved this final version of the manuscript.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
References
1. Vanni, G, Pellicciaro, M, Materazzo, M, Bruno, V, Oldani, C, Pistolese, CA, et al. Lockdown of Breast Cancer Screening for COVID-19: Possible Scenario. In Vivo (2020) 34(5):3047–53. doi:10.21873/invivo.12139
2.CBR/FEBRASGO/SBM. Joint Note from the Brazilian College of Radiology and Diagnostic Imaging (CBR), Brazilian Federation of Gynecology and Obstetrics Associations (Febrasgo) and Brazilian Society of Mastology (SBM) on Guidelines for Scheduling Breast Imaging Exams during the COVID-19 Pandemic (2020). Available at: https://cbr.org.br/wp-content/uploads/2020/03/NOTA-CONJUNTA-DO-CBR_FEBRASGO_SBMSOBRE-AS-ORIENTA%C3%87%C3%95ES-PARA-AGENDAMENTO-DOS-EXAMES-DE-DE-IMAGEM-DA-MAMA-DURANTE-A-PANDEMIA-DE-COVID-19-.pdf (Accessed October 9, 2022).
3.INCA. Technical Note - DIDEPRE/CONPREV/INCA Cancer Screening during the COVID-19 Pandemic, July 9 2020 (2020). Available at: https://www.inca.gov.br/sites/ufu.sti.inca.local/files/media/document/nota-tecnica-rastreamento-covid-didepre-09-jul-2020.pdf (Accessed October 9, 2022).
4.Pan American Health Organization/ World Health Organization. Considerations for the Reorganization of Cancer Services during the COVID-19 Pandemic, 26 May 2020 (2020). https://iris.paho.org/handle/10665.2/52263 (Accessed October 9, 2022).
5.INCA. Technical Note - DIDEPRE/CONPREV/INCA, 3 March 2020. Early Detection of Cancer during the Covid-19 Pandemic (2020). Available at: https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//nota_tecnica_deteccao_precoce_covid_marco_2020.pdf (Accessed October 9, 2022).
6. Pediconi, F, Galati, F, Bernardi, D, Belli, P, Brancato, B, Calabrese, M, et al. Breast Imaging and Cancer Diagnosis during the COVID-19 Pandemic: Recommendations from the Italian College of Breast Radiologists by SIRM. Radiol Med (2020) 125(10):926–30. doi:10.1007/s11547-020-01254-3
7.WHO. COVID-19 Significantly Impacts Health Services for Noncommunicable Diseases. World Health Organization (2020). Available at: https://www.who.int/news/item/01-06-2020-covid-19-significantly-impacts-health-services-for-noncommunicable-diseases (Accessed October 9, 2022).
8. Amorim, GL, Assad, DX, Ferrari, BL, Rosa, DD, Pereira, BP, Clara, RO, et al. Breast Oncology and the COVID-19 Pandemic: Recommendations from the Brazilian Society of Clinical Oncology (SBOC). Braz J Oncol (2020) 16:e20190024. doi:10.5935/2526-8732.20190024:
9. Azambuja, E, Trapani, D, Loibl, S, Delaloge, S, Senkus, E, Criscitiello, C, et al. ESMO Management and Treatment Adapted Recommendations in the COVID-19 Era: Breast Cancer. ESMO Open (2020) 5:e000793. doi:10.1136/esmoopen-2020-000793
10. Pendola, LG, Elizalde, R, Vargas, PS, Mallarino, JC, González, E, Parada, J, et al. Management of Non-invasive Tumours, Benign Tumours and Breast Cancer during the COVID-19 Pandemic: Recommendations Based on a Latin American Survey. Ecancermedicalscience (2020) 14:1115. doi:10.3332/ecancer.2020.1115
11. Ribeiro, CM, Correa, FM, and Migowski, A. Short-term Effects of the COVID-19 Pandemic on Cancer Screening, Diagnosis and Treatment Procedures in Brazil: a Descriptive Study, 2019-2020. Epidemiologia e Serviços de Saúde (2021) 31(1):e2021405. doi:10.1590/S1679-49742022000100010
12. Dinmohamed, AG, Cellamare, M, Visser, O, de Munck, L, Elferink, MAG, Westenend, PJ, et al. The Impact of the Temporary Suspension of National Cancer Screening Programmes Due to the COVID-19 Epidemic on the Diagnosis of Breast and Colorectal Cancer in the Netherlands. J Hematol Oncol (2020) 13(1):147. doi:10.1186/s13045-020-00984-1
13. Araujo, SEA, Leal, A, Centrone, AFY, Teich, VD, Malheiro, DT, Cypriano, AS, et al. Impact of COVID-19 Pandemic on Care of Oncological Patients: Experience of a Cancer center in a Latin American Pandemic Epicenter. Einstein (2020) 19:eAO6282–8. doi:10.31744/einstein_journal/2021AO6282
14. Freer, PE. The Impact of the COVID-19 Pandemic on Breast Imaging. Radiol Clin North Am (2021) 59(1):1–11. doi:10.1016/j.rcl.2020.09.008
15. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi:10.3322/caac.21660
16. Faria, RA, Ardisson, MD, Brito, GMS, Botelho, EVS, Pereira, SWA, Serafim, NR, et al. Therapeutic Guidelines Recommended for Breast Cancer Patients during the SARS-CoV-2 Pandemic: a Systematic Review of the Main Proposals Established by World Societies. Braz J Surg Clin Res (2020) 32(2):118–24.
17. Eijkelboom, AH, de Munck, L, Vrancken Peeters, MTFD, Broeders, MJM, Strobbe, LJA, Bos, MEMM, et al. Impact of the COVID-19 Pandemic on Diagnosis, Stage, and Initial Treatment of Breast Cancer in the Netherlands: a Population-Based Study. J Hematol Oncol (2021) 14(1):64. doi:10.1186/s13045-021-01073-7
18. Grimm, LJ, Lee, C, Rosenberg, RD, Burleson, J, Simanowith, M, Fruscello, T, et al. Impact of the COVID-19 Pandemic on Breast Imaging: an Analysis of the National Mammography Database. J Am Coll Radiol (2022) 19(8):919–34. doi:10.1016/j.jacr.2022.04.008
19. Koca, B, and Yildirim, M. Delay in Breast Cancer Diagnosis and its Clinical Consequences during the Coronavirus Disease Pandemic. J Surg Oncol (2021) 124(3):261–7. doi:10.1002/jso.26581
20. Mayo, M, Potugari, B, Bzeih, R, Scheidel, C, Carrera, C, and Shellenberger, RA. Cancer Screening during the COVID-19 Pandemic: a Systematic Review and Meta-Analysis. Mayo Clin Proc Innov Qual Outcomes (2021) 5(6):1109–17. doi:10.1016/j.mayocpiqo.2021.10.003
21. Migowski, A, Azevedo E Silva, G, Dias, MBK, Diz, MDPE, Sant’Ana, DR, and Nadanovsky, P. Guidelines for Early Detection of Breast Cancer in Brazil. II – New National Recommendations, Main Evidence, and Controversies. Cad Saude Publica (2018) 34(6):e00074817–16. doi:10.1590/0102-311X00074817
22.DATASUS. SUS-SIASUS Outpatient Information System. Department of Information Technology of SUS-DATASUS (2016). Available at: http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sia/cnv/qbuf.def (Accessed October 9, 2022).
23.DATASUS. Oncology. Treatment Monitoring Panel: Oncology Panel (2022). Available at: http://tabnet.datasus.gov.br/cgi/painel_onco/doc/painel_oncologia.pdf (Accessed October 9, 2022).
24.IBGE.Brazilian Institute of Geography and Statistics. Projections of the Population of Brazil and Federation Units by Sex and Age: 2010-2060 (2018). https://www.ibge.gov.br/estatisticas/sociais/populacao/9109-projecao-da-populacao.html?=&t=resultados (Accessed October 9, 2022).
25.ANS. National Agency for Supplemental Health. Health Insurance Coverage Rate (2022). Available at: http://www.ans.gov.br/anstabnet/cgi-bin/tabnet?dados/tabnet_tx.def (Accessed April 17, 2022).
26.INCA. Technical parameters for breast cancer screening. José Alencar Gomes da Silva National Cancer Institute. Rio de Janeiro: INCA (2021). Available at: http://controlecancer.bvs.br/ (Accessed April 17, 2022).
27. Rodrigues, DCN, Freitas-Junior, R, Rahal, RMS, da Silveira Corrêa, R, Gouveia, PA, Peixoto, JE, et al. Temporal Changes in Breast Cancer Screening Coverage provided under the Brazilian National Health Service between 2008 and 2017. BMC Public Health (2019) 19(1):959. doi:10.1186/s12889-019-7278-z
28.National Cancer Institute. Joinpoint Trend Analysis Software. Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute (2022). https://surveillance.cancer.gov/joinpoint/ (Accessed October 9, 2022).
29.International Business Machines Corporation. SPSS Statistics for Windows. Armonk, NY: IBM (2019).
30. Negrao, EMS, Cabello, C, Conz, L, Mauad, EC, Zeferino, LC, and Vale, DB. The COVID-19 Pandemic Impact on Breast Cancer Diagnosis: a Retrospective Study. Rev Bras Ginecol Obstet (2022) 44:871–7. doi:10.1055/s-0042-1749207
31. Bessa, JF. Breast Imaging Hindered during Covid-19 Pandemic in Brazil. Rev Saude Publica (2021) 55:202155. doi:10.11606/s1518-8787.2021055003375
32. Nogueira, MC, Fayer, VA, Correa, CSL, Guerra, MR, Stavola, B, Dos-Santos-Silva, I, et al. Inequities in Access to Mammographic Screening in Brazil. Cad Saude Publica (2019) 35(6):e00099817. doi:10.1590/0102-311X00099817
33. Rodrigues, DCN, Freitas-Junior, R, Rahal, RMS, Corrêa, RS, Peixoto, JE, Ribeiro, NV, et al. Difficult Access and Poor Productivity: Mammography Screening in Brazil. Asian Pac J Cancer Prev (2019) 20(6):1857–64. doi:10.31557/APJCP.2019.20.6.1857
34. Abrahão, KS, Bergmann, A, Aguiar, SS, and Thuler, LC. Determinants of Advanced Stage Presentation of Breast Cancer in 87,969 Brazilian Women. Maturitas (2015) 82(4):365–70. doi:10.1016/j.maturitas.2015.07.021
35. Oliveira, NPD, de Camargo Cancela, M, Martins, LFL, and de Souza, DLB. A Multilevel Assessment of the Social Determinants Associated with the Late Stage Diagnosis of Breast Cancer. Sci Rep (2021) 11(1):2712. doi:10.1038/s41598-021-82047-0
36. Toss, A, Isca, C, Venturelli, M, Nasso, C, Ficarra, G, Bellelli, V, et al. Two-month Stop in Mammographic Screening Significantly Impacts on Breast Cancer Stage at Diagnosis and Upfront Treatment in the COVID Era. ESMO Open (2021) 6(2):100055. doi:10.1016/j.esmoop.2021.100055
37. Kang, YJ, Baek, JM, Kim, YS, Jeon, YW, Yoo, TK, Rhu, J, et al. Impact of the COVID-19 Pandemic on the Diagnosis and Surgery of Breast Cancer: a Multi-Institutional Study. J Breast Cancer (2021) 24(6):491–503. doi:10.4048/jbc.2021.24.e55
38. Bonadio, RC, Messias, AP, Moreira, OA, Leis, LV, Orsi, BZ, Testa, L, et al. Impact of the COVID-19 Pandemic on Breast and Cervical Cancer Stage at Diagnosis in Brazil. Ecancermedicalscience (2021) 15:1299. doi:10.3332/ecancer.2021.1299
39. Yong, JH, Mainprize, JG, Yaffe, MJ, Ruan, Y, Poirier, AE, Coldman, A, et al. The Impact of Episodic Screening Interruption: COVID-19 and Population-Based Cancer Screening in Canada. J Med Screen (2021) 28(2):100–7. doi:10.1177/0969141320974711
Keywords: breast cancer, COVID-19, Brazil, breast cancer screening, clinical staging of breast cancer
Citation: Rocha AFBM, Freitas-Junior R, Ferreira GLR, Rodrigues DCN and Rahal RMS (2023) COVID-19 and Breast Cancer in Brazil. Int J Public Health 68:1605485. doi: 10.3389/ijph.2023.1605485
Received: 13 October 2022; Accepted: 24 February 2023;
Published: 03 March 2023.
Edited by:
Aylene Bousquat, University of São Paulo, BrazilCopyright © 2023 Rocha, Freitas-Junior, Ferreira, Rodrigues and Rahal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aline Ferreira Bandeira Melo Rocha, YWxpbmViYW5kZWlyYWdlcmlhdHJhQGdtYWlsLmNvbQ==
 Aline Ferreira Bandeira Melo Rocha
Aline Ferreira Bandeira Melo Rocha Ruffo Freitas-Junior2
Ruffo Freitas-Junior2