Abstract
Objective: To investigate the effect of exposure to multiple ambient air pollutants during pregnancy on the risk of children being born small for gestational age (SGA).
Methods: An Air Pollution Score (APS) was constructed to assess the effects of being exposed to six air pollutants simultaneously, PM2.5, PM10, SO2, NO2, CO, and O3 (referred to as joint exposure). A logistic regression model was applied to estimate the associations of APS and SGA.
Results: The adjusted odds ratios (ORs) of SGA per 10 ug/m3 increased in APS during the first and second trimesters and the entire pregnancy were 1.003 [95% confidence intervals (CIs): 1.000, 1.007], 1.018 (1.012, 1.025), and 1.020 (1.009, 1.031), respectively. The ORs of SGA for each 10 μg/m3 elevated in APS during the whole pregnancy were 1.025 (1.005, 1.046) for mothers aged over 35 years old vs. 1.018 (1.005, 1.031) for mothers aged under 35 years old. Women who were pregnant for the first time were more vulnerable to joint ambient air pollution.
Conclusion: In summary, the results of the present study suggested that joint exposure to ambient air pollutants was associated with the increment in the risks of SGA.
Introduction
Small for gestational age (SGA) is an indicator of fetal development. It applies when infants are born with a birth weight below the 10th percentile for the average weight of newborns of the same gestational age [1]. The prevalence of SGA in developed regions of Europe and America was approximately 10% in 2017 [2]. A previous study estimated that in 2010 more than 32 million SGA infants in low- and middle-income countries and the number of SGA births in China was 1,072,100 (uncertainty intervals: 648,300-1,817,600), meaning China ranked fifth among the top ten countries with the highest numbers of SGA infants [3]. Lower birth weight is one of the major risk factors for neonatal death [4, 5]. In addition, SGA is associated with cognitive impairment in childhood and the development of obesity, type 2 diabetes mellitus, and cardiovascular diseases in adulthood [6, 7].
Air pollution has been an alarming public health problem worldwide in recent years [8]. Emerging studies have shown that short- and long-term exposure to air pollution is associated with elevated mortality and morbidity of various diseases [9, 10]. The relationship between air pollution and SGA had been extensively assessed in recent years [11, 12]. A study conducted in Huangshi, China found that fine particulate matter (PM2.5) and inhalable particles (PM10) were positively linked with increased risks of SGA during the entire pregnancy [13]. In the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Consecutive Pregnancy Study, the significant effects of PM2.5, PM10, nitrogen dioxide (NO2), sulfur dioxide (SO2), and carbon monoxide (CO) on SGA were found in the third trimester [14]. However, the overwhelming majority of research to date has applied single-pollutant models, which often ignore the fact that multiple people are exposed to ambient air pollution simultaneously. The combined health effect of particulate matter and gaseous pollutants might be widely divergent from that of individual air pollutants [15, 16]. Therefore, we propose a novel indicator [17], the Air Pollution Score (APS), to aid in comprehensively considering the effects of PM2.5, PM10, NO2, SO2, CO, and ozone (O3), in evaluating the associations of mixed exposure to air pollution and SGA.
This study applied logistic regression models to estimate the associations between joint exposure to multiple ambient air pollutants and SGA in Wuhan, China. We also performed subgroup analyses to explore potentially susceptible populations and the times of year when people are most vulnerable.
Methods
Study Design and Population
Wuhan Children’s Hospital is a large-scale specialized hospital located in the Jiang’an district of Wuhan, China. This area is one of the central urban areas of Wuhan city, serving pregnant women and the delivery needs of the whole city (Supplementary Figure S1). Data on mothers and live newborns were collected from Wuhan Children’s Hospital from 1 January 2017, to 30 June 2021. After screening by inclusion and exclusion criteria, a total of 31,283 gravidas and their offspring were involved in the study (Figure 1). Their geographical distributions are shown in Figure 2. We obtained variables of interest from the hospital’s delivery register, including each gravida’s residential address and duration, work status, educational attainment, maternal age, the number of pregnancies and parity, high-risk factors during pregnancy (for example, placental abruption, placenta previa, gestational hypertension, preeclampsia, eclampsia, oligohydramnios, and gestational diabetes, etc.), gestational age, date of delivery, birth weight and sex of newborns. Furthermore, we also deduced gravidas’ conception seasons [warm (April to September) and cold (October to March of the next year)] based on the date of delivery and gestational age.
FIGURE 1
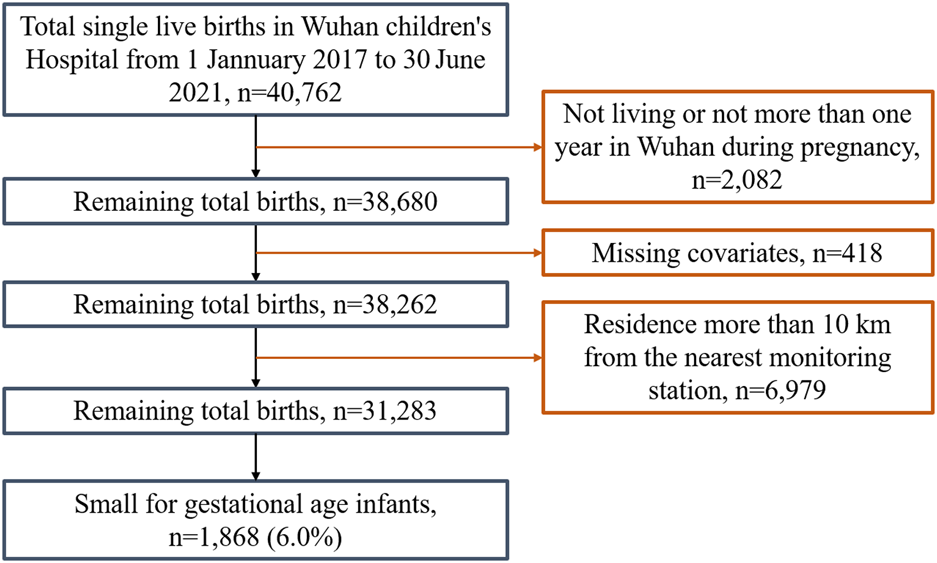
Flow chart of the study population selection (Wuhan, China, 2022).
FIGURE 2
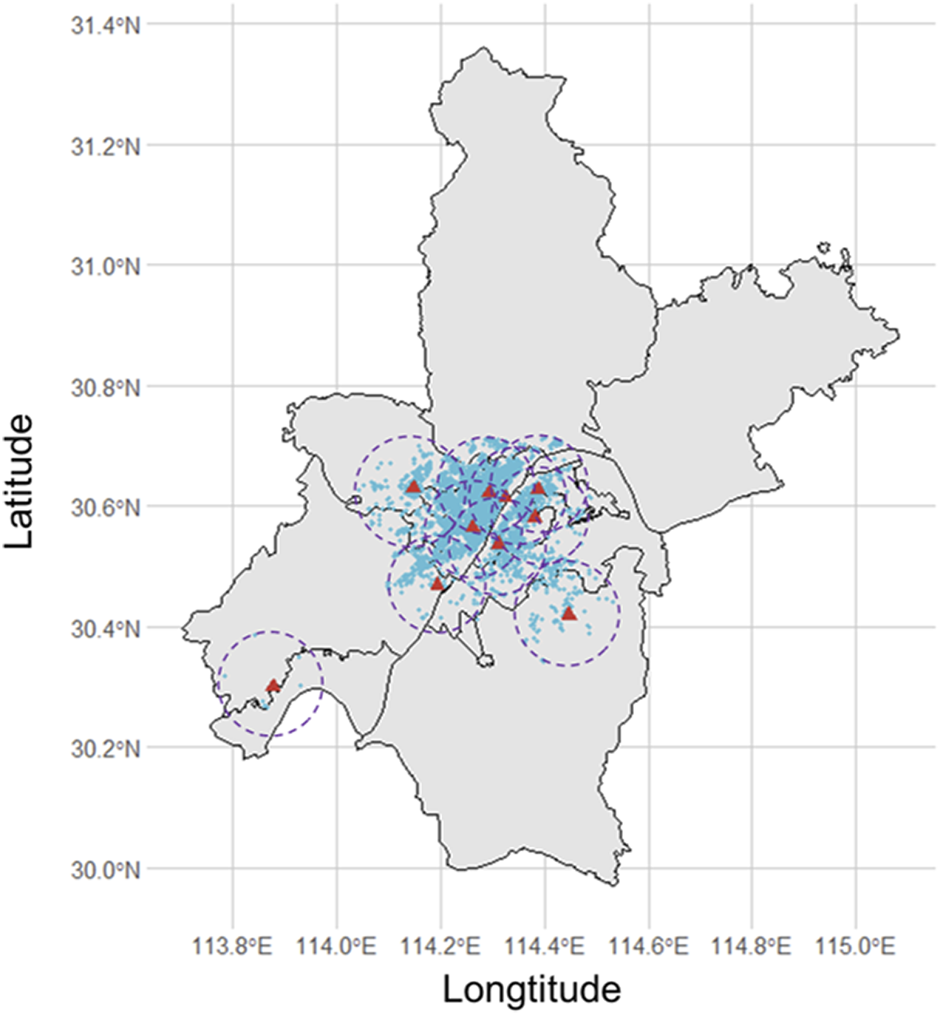
The distribution of pregnant women whose home addresses are less than 10 km from the nearest monitoring station (Wuhan, China, 2022).
Assessment of SGA
The weight of the newborns was measured within 1 hour after birth. SGA was defined as infants with a birth weight lower than the 10th percentile of the average weight at the same gestational age [18]. Compared with the traditional indicator (low birth weight, LBW), SGA can more accurately reflect the development of the infant by considering development time in the womb. According to growth standard curves of the birth weights of Chinese newborns of different gestation [19], the incidence rate of SGA was approximately 6.0% in this study.
Definition of the Air Pollution Score
The daily mean concentrations of PM2.5, PM10, SO2, NO2, CO, and O3 were collected from 10 national environmental monitoring stations based in the Wuhan Municipal Bureau of Ecological Environment (http://hbj.wuhan.gov.cn/hjsj/). The concentration of air pollution published by the environmental monitoring station closest to the home address of gravida was used as the individual exposure level (Figure 1).
APS is a novel indicator for assessing the joint effects of being exposed to multiple ambient air pollutants simultaneously and the health outcomes that result from it [17]. In the current study, the air pollution score was calculated by considering concentrations of six air pollutants, weighted by multivariable-adjusted risk estimates (β coefficients) on SGA. The formula was as follows:Where and were the regression coefficients calculated from each single-pollutant model and were the concentration of each air pollutant. The higher APS of the pregnant woman indicated that they had been exposed to higher joint ambient air pollutants.
Statistical Analysis
According to previous epidemiological studies [20], a pregnancy period is divided into the first trimester (1–12 weeks), second trimester (13–26 weeks), and third trimester (27 weeks to birth). A two-stage analysis strategy was developed to investigate the associations between joint ambient air pollution and SGA. In the first stage, the links between six air pollutants and SGA were assessed by performing multiple logistic regression in separate models, and then APS was calculated. At the second stage, the effects of APS on SGA were evaluated to reflect the hazards of mixed air pollution exposure. Several potential confounders were selected and adjusted in these models, including age [year old (<35, 35+)], pregnancy (=1, >1), parity (=1, >1), educational attainment [year (≤9, 10–12, ≥13)], work status (yes, no), high-risk factor during pregnancy (yes, no) and neonatal sex (boy, girl). Moreover, we separated APS into five quintiles (Q1, Q2, Q3, Q4, and Q5) and estimate the ORs compared with the first quintiles (Q1) to evaluate the potential linear trend between APS and SGA. Subgroup analyses were stratified by age, pregnancy, parity, and conception season to detect vulnerable populations and periods. To assess the robustness of the association between APS and SGA, we conducted several sensitivity analyses: 1) re-calculating APS removing one air pollutant at a time; 2) assessment of SGA using internal standard; 3) co-adjusting for two trimesters in the same model; 4) co-controlling for two air pollutants in the same model.
Consistent with previous studies, the effects of air pollution and APS on SGA were reported odds ratio (ORs) with 95% confidence intervals (CIs). All statistical analyses were performed using R software (version 4.0.3), All P-values for the tests were two-sided and P-values < 0.05 were considered statistically significant.
Results
Table 1 shows the characteristics of mothers and newborns in Wuhan, China. In both the case group and control group, lying-in women who were aged under 35 years old, had primiparity, higher educational level, were working, and had high-risk factors, and those who gave birth to boys accounted for more than half of the total participants. However, a higher proportion of first-time pregnant mothers were observed in the SGA group compared with the normal group (57.3% vs. 45.9%). Table 2 shows the adjusted odds ratios (ORs) and 95% CIs for SGA associated with a 10 ug/m3 increase in air pollutants during different exposure periods. Exposure to SO2 appeared the strongest effects on SGA in the second trimester [OR = 1.320, 95% CIs (1.110, 1.570)].
TABLE 1
| Control (n = 29,415) | SGA (n = 1868) | |
|---|---|---|
| Age (years), n (%) | ||
| <35 | 18,522 (63.0%) | 1,321 (70.7%) |
| 35+ | 10,893 (37.0%) | 547 (29.3%) |
| Pregnancy, n (%) | ||
| =1 | 13,515 (45.9%) | 1,071 (57.3%) |
| >1 | 15,900 (54.1%) | 797 (42.7%) |
| Parity, n (%) | ||
| =1 | 18,914 (64.3%) | 1,465 (78.4%) |
| >1 | 10,501 (35.7%) | 403 (21.6%) |
| Educational attainment (years), n (%) | ||
| ≤9 | 5,363 (18.2%) | 324 (17.3%) |
| 10–12 | 2,164 (7.4%) | 133 (7.1%) |
| ≥13 | 21,888 (74.4%) | 1,411 (75.5%) |
| Work status, n (%) | ||
| Yes | 17,307 (58.8%) | 1,098 (58.8%) |
| No | 12,108 (41.2%) | 770 (41.2%) |
| High-risk factor, n (%) | ||
| Yes | 21,652 (73.6%) | 1,334 (71.4%) |
| No | 7,763 (26.4%) | 534 (28.6%) |
| Neonatal sex, n (%) | ||
| Boy | 15,595 (53.0%) | 1,062 (56.9%) |
| Girl | 13,820 (47.0%) | 806 (43.1%) |
The characteristics of mothers and newborns (Wuhan, China, 2022).
TABLE 2
| Trimester 1 | Trimester 2 | Trimester 3 | Entire pregnancy | |
|---|---|---|---|---|
| PM2.5 | 1.009 (0.982, 1.036) | 1.077 (1.049, 1.106) | 0.988 (0.962, 1.014) | 1.116 (1.053, 1.181) |
| PM10 | 1.009 (0.986, 1.033) | 1.062 (1.037, 1.087) | 1.010 (0.987, 1.033) | 1.069 (1.030, 1.109) |
| SO2 | 1.045 (0.882, 1.237) | 1.320 (1.110, 1.570) | 0.977 (0.815, 1.172) | 1.261 (0.969, 1.640) |
| NO2 | 1.034 (0.989, 1.080) | 1.087 (1.040, 1.136) | 0.970 (0.929, 1.013) | 1.085 (1.008, 1.169) |
| CO | 1.003 (1.000, 1.006) | 1.005 (1.002, 1.008) | 1.000 (0.997, 1.002) | 1.001 (1.000, 1.002) |
| O3 | 0.977 (0.953, 1.001) | 0.943 (0.921, 0.967) | 1.035 (1.010, 1.060) | 0.918 (0.866, 0.973) |
The odds ratios and 95% confidence intervals for small for gestational age infants associated with a 10 μg per cubic meter increase in air pollutants (Wuhan, China, 2022).
Table 3 exhibits adjusted odds ratios (ORs) and 95% confidence intervals for the associations of SGA and APS. A 10 ug/m3 increase in APS was positively associated with elevated risk of SGA, with corresponding ORs of 1.018 (95% CIs: 1.012, 1.025) and 1.020 (95% CIs: 1.009, 1.031) in the second trimester and the entire pregnancy, respectively. Compared with the first quintile of APS in different pregnancy periods. There was a significant relationship between APS and SGA detected in the second trimester [Q5 vs. Q1, OR = 1.427 (95% CIs: 1.231, 1.653)] and entire pregnancy [Q5 vs. Q1, OR = 1.346 (95% CIs: 1.162, 1.558)]. In general, the higher the concentrations of APS, the larger their effect estimates on SGA (p for trend <0.05).
TABLE 3
| Air pollution score (quintiles) | ORs for per 10 ug/m3 increase | p for trend | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Trimester 1 | 1 | 1.042 (0.893, 1.216) | 1.247 (1.075, 1.447) | 1.206 (1.038, 1.401) | 1.105 (0.949, 1.288) | 1.003 (1.000, 1.007) | 0.046 |
| Trimester 2 | 1 | 1.054 (0.902, 1.232) | 1.118 (0.959, 1.304) | 1.133 (0.972, 1.320) | 1.427 (1.231, 1.653) | 1.018 (1.012, 1.025) | <0.001 |
| Trimester 3 | 1 | 1.157 (1.001, 1.336) | 1.016 (0.876, 1.179) | 1.006 (0.868, 1.168) | 0.862 (0.738, 1.005) | 0.998 (0.996, 0.999) | 0.013 |
| Entire pregnancy | 1 | 1.129 (0.972, 1.311) | 0.987 (0.845, 1.152) | 0.993 (0.850, 1.160) | 1.346 (1.162, 1.558) | 1.020 (1.009, 1.031) | 0.003 |
The adjusted odd ratios and 95% confidence intervals for air pollution scores with the risk of small for gestational age infants in Wuhan (Wuhan, China, 2022).
Note: Q, quintile.
Table 4 compares the adjusted ORs per 10 ug/m3 increment of APS in different subgroups during the four exposure windows. Pregnant women with advanced maternal age seemed to be more susceptible to exposure to mixed air pollutants than younger women. The ORs of SGA per 10 ug/m3 elevated in APS during the second trimester and entire pregnancy were 1.022 (95% CIs: 1.010, 1.033) for mothers aged over 35 years old and 1.016 (95% CIs: 1.008, 1.024) for mothers aged under 35 years old, and 1.025 (95% CIs: 1.005, 1.046) vs. 1.018 (95% CIs: 1.005, 1.031), respectively. Results showed that women experiencing their first pregnancy were more vulnerable to joint ambient air pollution than women who have had multiple pregnancies. For the conception season, the increment of APS related to an increase in the risk of SGA corresponded with the warm season only.
TABLE 4
| Trimester 1 | Trimester 2 | Trimester 3 | Entire pregnancy | |
|---|---|---|---|---|
| Age (years) | ||||
| <35 | 1.004 (1.000, 1.009) | 1.016 (1.008, 1.024) | 0.998 (0.996, 1.000) | 1.018 (1.005, 1.031) |
| 35+ | 1.002 (0.995, 1.009) | 1.022 (1.010, 1.033) | 0.998 (0.995, 1.001) | 1.025 (1.005, 1.046) |
| Pregnancy | ||||
| =1 | 1.005 (1.000, 1.010) | 1.020 (1.011, 1.028) | 0.997 (0.995, 0.999) | 1.025 (1.010, 1.040) |
| >1 | 1.001 (0.995, 1.007) | 1.016 (1.006, 1.027) | 0.999 (0.996, 1.001) | 1.014 (0.998, 1.031) |
| Parity | ||||
| =1 | 1.005 (1.000, 1.009) | 1.018 (1.010, 1.025) | 0.997 (0.995, 0.999) | 1.019 (1.006, 1.031) |
| >1 | 0.999 (0.991, 1.007) | 1.019 (1.005, 1.034) | 1.001 (0.998, 1.005) | 1.026 (1.003, 1.050) |
| Season | ||||
| Warm | 1.018 (1.011, 1.025) | 1.026 (1.017, 1.036) | 0.993 (0.991, 0.996) | 1.029 (1.013, 1.046) |
| Cold | 1.006 (0.998, 1.013) | 1.004 (0.992, 1.016) | 0.998 (0.995, 1.002) | 1.007 (0.991, 1.023) |
The adjusted odd ratios and 95% confidence intervals for a 10 μg per cubic meter increase in air pollution scores with the risk of small for gestational age infants in Wuhan (Wuhan, China, 2022).
After re-calculating APS by removing one air pollutant at a time, the links between APS and SGA were still statistically significant in the second trimester and the entire pregnancy (Supplementary Table S1). We re-estimated the associations between SGA (internal standard, as seen in Supplementary Table S2) and air pollutants. Compared with previous results, the associations still exist, although the impact was slightly smaller (Supplementary Table S3). In two-trimester and two-pollutant models, the relationship between air pollution and SGA did not change significantly (Supplementary Tables S4, S5). The several sensitivity analyses showed our results were robust.
Discussion
In this study, we used APS to estimate the effect of joint exposure to ambient air pollutants on SGA by unconditional logistic regression models. We found significant positive associations between APS-SGA in the first, second, and third trimesters, as well as for the entire pregnancy. Compared with the first quintile of APS, the effect estimates were strongest in the fifth quintile. Gravidas, being aged over 35 years old, multiparas, women experiencing their first pregnancy, and conception taking place in the warm season were shown to make pregnancies more vulnerable to mixed air pollution during the second trimester and whole pregnancy. These findings contribute to the existing body of knowledge on the relationship between mixed air pollution and SGA and provide valuable epidemiological evidence for policymakers and will help better maternal and child health.
A number of studies have revealed that exposure to air pollution during pregnancy increases the risk of SGA [21, 22]. For example, a 1 ug/m3 increase in PM2.5 during the second trimester corresponded with a 1.9% (95% CIs: 0.9%, 2.8%) increased incidence of SGA [13]. The third quartile of PM10 in the whole pregnancy compared with the first quartile was linked with SGA [OR = 1.38 (95% CIs: 1.00, 1.90)] [23]. The adjusted ORs for SGA associated with per interquartile range (IQR) increased in SO2, NO2, and O3 during the entire pregnancy were 1.02 (95% CIs: 1.01, 1.03), 1.08 (95% CIs: 1.04, 1.12), and 1.14 (95% CIs: 1.11, 1.17), respectively [12]. A 1 part per million (ppm) increase in the concentration of CO in the first month of pregnancy was significantly related to SGA [OR = 1.06 (95% CIs: 1.01, 1.10)] [24]. However, in the current paper, we found negative correlations of O3. Possible explanations is the different ethnicities of patients and the fact that the combination of air pollution in specific regions differed. Animal experiments using an ozone exposure chamber are warranted to investigate the associations between O3 and SGA.
In recent years, more research attention has been paid to the health effects of mixed pollutant exposure due to the high correlation between them and the fact that they might be emitted simultaneously from the same sources [25, 26]. However, few studies have estimated the joint association between ambient air pollutants and SGA. We comprehensively constructed a novel indicator, APS, reflecting the combined effects of PM2.5, PM10, SO2, NO2, CO, and O3, to evaluate the association between joint exposure to air pollutants and SGA. Although NO2, CO, and O3 appeared non-positive effects on SGA in the single-pollutant models, we found that APS was significantly associated with an elevated risk of SGA in each exposure window. Therefore, APS might have the capacity to provide more comprehensive measures of health assessment than individual air pollutants. Prior studies had utilized similar methods by accounting for a combination of common coexisting pollutants to assess the combined effects of multi-pollutant exposures on health outcomes [27, 28]. Hong et al. proposed an index calculated by summing each air pollutant concentration divided by its mean and observed that the new index was more strongly associated with all-cause mortality than individual air pollutants [28]. An analogous algorithm, environmental risk score (ERS) was also used to estimate the relationships between joint exposure to environmental pollutants and serum lipid levels in people in the US [29]. A statistical approach to comprehensively summarizing air pollutant concentrations could efficiently evaluate the combined effects of pollutants as they are highly correlated [17].
Previous studies have indicated that prenatal exposure to air pollution is associated with a smaller head circumference, shorter body length, and lower weight in newborns [30–32]. The potential biological mechanism of air pollution on SGA remains unclear. However, there were several theories worth mentioning, including: 1) that air pollution might trigger oxidative stress and inflammatory reaction, reducing nutrition and the exchange of gases in the placenta and inducing endocrine disorders in the maternal body [33]; 2) prenatal exposure to air pollution could increase maternal susceptibility to infections, impairing fetal growth [34]; and 3) exposure to air pollution is capable of reducing the content of mitochondrial DNA (mtDNA), which has been related to lower infant birth weight and more muscular oxidative stress [35, 36]. The potential mechanism of joint exposure to air pollutants and increased risks of SGA is still unknown. We speculated that there might be additive effects in various air pollutants on SGA because of their similar biological function pathways, such as oxidative stress and inflammatory reaction.
We observed that when the maternal age is over 35 years old the pregnancy seems to be more vulnerable to APS in the second trimester and whole pregnancy, which can be explained due to the problem of hypoxia in older pregnant women being more prominent due to the increase in air pollution levels and umbilical artery vasoconstriction [37, 38]. Younger women might generally be more capable of relieving the oxidative stress induced by air pollution [39]. Slightly stronger associations between APS and SGA were found in women who were having their first pregnancy than those who have had multiple pregnancies, during the entire pregnancy, with corresponding ORs of 1.025 (95% CIs: 1.010, 1.040) vs. 1.014 (95% CIs: 0.998, 1.031). Prior studies have also revealed that women with a history of pregnancy and childbirth were associated with an increase in the birth weights of newborns [40, 41]. Prefumo et al. found that permanent changes in maternal blood vessels might persist after a successful pregnancy and that these changes in the physiological structure also changed the hemodynamics of women during the second pregnancy, which was more conducive to the material exchange between mother and fetus [42]. Previous studies have evidenced that the placental efficiency of primipara is relatively low, which affects the development of the surface density of the microcotyledon; whereas with an increase in the number of parities, the surface density elevates accordingly [43–45], which might mitigate the hazards of air pollution to a certain degree. The specific mechanism of how both parity and APS influence neonatal birth weight needs to be further explored. In this paper, we found that when women conceived in the warm season they were more susceptible to APS than in the cold season. Similar results were found in a study conducted in Guangzhou, China [12]. Wang et al. observed that an IQR increase in PM2.5, PM10, NO2, SO2, and O3 significantly elevated SGA incidence among women whose conception season was summer or fall [12]. However, a study conducted in Canada found there was no seasonal pattern between air pollution and SGA [32], meaning weather conditions might only partly explain the difference. Wuhan and Guangzhou belong to subtropical oceanic monsoon climates, and women conceived in the warm season experience relatively cold weather during pregnancy. A previous study pointed out that people living in Wuhan and Guangzhou were more vulnerable to cold weather than those in the cities of northern China [46]. Therefore, simultaneous exposure to lower temperatures and humidity might strengthen the adverse effect of air pollution on SGA. Besides, the lower duration of sunshine in the cold season has been associated with lower maternal vitamin D levels, and vitamin D deficiency was a risk factor for low birth weight in infants [47].
According to the information we currently have, this is the first study to explore the associations between joint exposure to ambient air pollutants and SGA in China. These findings deepen our understanding of the impact of mixed air pollution and indicate that new prevention strategies to curb various air pollutants together are needed. There are several limitations of this study that must be acknowledged: 1) data on air pollution reported by the environmental monitoring station closest to the home address of gravida was used to measure individual exposure, which could lead to misclassifications to some extent; 2) we regarded air pollutants as continuous variables when constructing APS, however, the link between air pollution and SGA might not be linear; 3) we did not adjust for some potential confounders such as work hazards, smoking, drinking, diet etc., due to data on these not being available; 4) this study was conducted in Wuhan, so caution should be taken when conclusions were extended to other regions; 5) although choosing which hospital to give birth is the decision of the patient, there might be selection bias because the results only relate to one hospital; 6) the effects of O3 might be confounded by other air pollutants, so animal experiments using an ozone exposure chamber are warranted to investigate the true associations between O3 and SGA.
Conclusion
In summary, our study suggested that joint exposure to ambient air pollutants including PM2.5, PM10, SO2, NO2, CO, and O3, evaluated as APS, were associated with an increase in risks of SGA. Women who were of advanced maternal age, primipara, first pregnancy, and conceived in the warm season were more susceptible to APS. Consequently, our results might provide valuable epidemiological evidence for policymakers and public health departments to comprehensively curb air pollution and protect neonatal health.
Statements
Ethics statement
This study was approved by the Ethics Committee of Wuhan Children’s Hospital (2021R139-F01).
Author contributions
WZ and YaZ conceived and designed the study; FZ and YuZ collected and cleaned data; FZ and XuZ performed data analysis and drafted the manuscript. SZ, XuZ, XiZ, GZ, and TL helped revise the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by grants from the Fundamental Research Funds for the Central Universities (204202021kf0044) and Post-doctoral innovation research post in Hubei Province (211000011).
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2022.1605391/full#supplementary-material
References
1.
Ding G Tian Y Zhang Y Pang Y Zhang JS Zhang J . Application of a Global Reference for Fetal-Weight and Birthweight Percentiles in Predicting Infant Mortality. BJOG (2013) 120(13):1613–21. 10.1111/1471-0528.12381
2.
Francis A Hugh O Gardosi J . Customized vs INTERGROWTH-21(st) Standards for the Assessment of Birthweight and Stillbirth Risk at Term. Am J Obstet Gynecol (2018) 218(2S):S692–S699. 10.1016/j.ajog.2017.12.013
3.
Lee ACC Katz J Blencowe H Cousens S Kozuki N Vogel JP et al National and Regional Estimates of Term and Preterm Babies Born Small for Gestational Age in 138 Low-Income and Middle-Income Countries in 2010. Lancet Glob Health (2013) 1(1):E26–E36. 10.1016/S2214-109X(13)70006-8
4.
Martin LJ Sjors G Reichman B Darlow BA Morisaki N Modi N et al Country-Specific vs. Common Birthweight-For-Gestational Age References to Identify Small for Gestational Age Infants Born at 24-28 Weeks: An International Study. Paediatr Perinat Epidemiol (2016) 30(5):450–61. 10.1111/ppe.12298
5.
Ray JG Park AL Fell DB . Mortality in Infants Affected by Preterm Birth and Severe Small-For-Gestational Age Birth Weight. Pediatrics (2017) 140(6):e20171881. 10.1542/peds.2017-1881
6.
Mericq V Martinez-Aguayo A Uauy R Iniguez G Van der Steen M Hokken-Koelega A . Long-term Metabolic Risk Among Children Born Premature or Small for Gestational Age. Nat Rev Endocrinol (2017) 13(1):50–62. 10.1038/nrendo.2016.127
7.
Sacchi C Marino C Nosarti C Vieno A Visentin S Simonelli A . Association of Intrauterine Growth Restriction and Small for Gestational Age Status with Childhood Cognitive Outcomes: A Systematic Review and Meta-Analysis. JAMA Pediatr (2020) 174(8):772–81. 10.1001/jamapediatrics.2020.1097
8.
Fuller R Landrigan PJ Balakrishnan K Bathan G Bose-O'Reilly S Brauer M et al Pollution and Health: a Progress Update. Lancet Planet Health (2022) 6:e535–e547. 10.1016/S2542-5196(22)00090-0
9.
Liu C Chen R Sera F Vicedo-Cabrera AM Guo Y Tong S et al Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N Engl J Med (2019) 381(8):705–15. 10.1056/NEJMoa1817364
10.
Shi L Steenland K Li H Liu P Zhang Y Lyles RH et al A National Cohort Study (2000-2018) of Long-Term Air Pollution Exposure and Incident Dementia in Older Adults in the United States. Nat Commun (2021) 12(1):6754. 10.1038/s41467-021-27049-2
11.
Bijnens EM Derom C Gielen M Winckelmans E Fierens F Vlietinck R et al Small for Gestational Age and Exposure to Particulate Air Pollution in the Early-Life Environment of Twins. Environ Res (2016) 148:39–45. 10.1016/j.envres.2016.03.006
12.
Wang Q Benmarhnia T Li C Knibbs LD Bao J Ren M et al Seasonal Analyses of the Association between Prenatal Ambient Air Pollution Exposure and Birth Weight for Gestational Age in Guangzhou, China. Sci Total Environ (2019) 649:526–34. 10.1016/j.scitotenv.2018.08.303
13.
Hao J Zhang F Chen D Liu Y Liao L Shen C et al Association between Ambient Air Pollution Exposure and Infants Small for Gestational Age in Huangshi, China: a Cross-Sectional Study. Environ Sci Pollut Res Int (2019) 26(31):32029–39. 10.1007/s11356-019-06268-7
14.
Nobles CJ Grantz KL Liu D Williams A Ouidir M Seeni I et al Ambient Air Pollution and Fetal Growth Restriction: Physician Diagnosis of Fetal Growth Restriction versus Population-Based Small-For-Gestational Age. Sci Total Environ (2019) 650:2641–7. 10.1016/j.scitotenv.2018.09.362
15.
Mauderly JL Samet JM . Is There Evidence for Synergy Among Air Pollutants in Causing Health Effects?Environ Health Perspect (2009) 117(1):1–6. 10.1289/ehp.11654
16.
Pearce JL Neelon B Bozigar M Hunt KJ Commodore A Vena J . Associations between Multipollutant Day Types and Select Cardiorespiratory Outcomes in Columbia, South Carolina, 2002 to 2013. Environ Epidemiol (2018) 2(4):e030. 10.1097/EE9.0000000000000030
17.
Wang MY Zhou T Song Y Li X Ma H Hu Y et al Joint Exposure to Various Ambient Air Pollutants and Incident Heart Failure:a Prospective Analysis in UK Biobank. Eur Heart J (2021) 42(16):1582–91. 10.1093/eurheartj/ehaa1031
18.
Bergstra AD Brunekreef B Burdorf A . The Influence of Industry-Related Air Pollution on Birth Outcomes in an Industrialized Area. Environ Pollut (2021) 269:115741. 10.1016/j.envpol.2020.115741
19.
Li H . Growth Standard Curves of Birth Weight, Length and Head Circumference of Chinese Newborns of Different Gestation. Zhonghua Er Ke Za Zhi (2020) 58(9):738–46. 10.3760/cma.j.cn112140-20200316-00242
20.
Wu J Ren C Delfino RJ Chung J Wilhelm M Ritz B . Association between Local Traffic-Generated Air Pollution and Preeclampsia and Preterm Delivery in the South Coast Air Basin of California. Environ Health Perspect (2009) 117(11):1773–9. 10.1289/ehp.0800334
21.
Smith RB Fecht D Gulliver J Beevers SD Dajnak D Blangiardo M et al Impact of London's Road Traffic Air and Noise Pollution on Birth Weight: Retrospective Population Based Cohort Study. Bmj-British Med J (2017) 359:j5299. 10.1136/bmj.j5299
22.
Lee PC Wu CD Tsai HJ Tsai HY Lin SH Wu CK et al Residential Greenness and Birth Outcomes: Evaluating the Mediation and Interaction Effects of Particulate Air Pollution. Ecotoxicology Environ Saf (2021) 211:111915. 10.1016/j.ecoenv.2021.111915
23.
van den Hooven EH Pierik FH de Kluizenaar Y Willemsen SP Hofman A van Ratingen SW et al Air Pollution Exposure during Pregnancy, Ultrasound Measures of Fetal Growth, and Adverse Birth Outcomes: a Prospective Cohort Study. Environ Health Perspect (2012) 120(1):150–6. 10.1289/ehp.1003316
24.
Liu SL Krewski D Shi Y Chen Y Burnett RT . Association between Gaseous Ambient Air Pollutants and Adverse Pregnancy Outcomes in Vancouver, Canada. Environ Health Perspect (2003) 111(14):1773–8. 10.1289/ehp.6251
25.
Dominici F Peng RD Barr CD Bell ML . Protecting Human Health from Air Pollution: Shifting from a Single-Pollutant to a Multipollutant Approach. Epidemiology (2010) 21(2):187–94. 10.1097/EDE.0b013e3181cc86e8
26.
Johns DO Stanek LW Walker K Benromdhane S Hubbell B Ross M et al Practical Advancement of Multipollutant Scientific and Risk Assessment Approaches for Ambient Air Pollution. Environ Health Perspect (2012) 120(9):1238–42. 10.1289/ehp.1204939
27.
Stieb DM Burnett RT Smith-Doiron M Brion O Shin HH Economou V . A New Multipollutant, No-Threshold Air Quality Health index Based on Short-Term Associations Observed in Daily Time-Series Analyses. J Air Waste Manag Assoc (2008) 58(3):435–50. 10.3155/1047-3289.58.3.435
28.
Hong YC Leem JH Ha EH Christiani DC . PM(10) Exposure, Gaseous Pollutants, and Daily Mortality in Inchon, South Korea. Environ Health Perspect (1999) 107(11):873–8. 10.1289/ehp.99107873
29.
Park SK Tao Y Meeker JD Harlow SD Mukherjee B . Environmental Risk Score as a New Tool to Examine Multi-Pollutants in Epidemiologic Research: an Example from the NHANES Study Using Serum Lipid Levels. PLoS One (2014) 9(6):e98632. 10.1371/journal.pone.0098632
30.
MoghaddamHosseini V Dowlatabadi A Najafi ML Ghalenovi M Pajohanfar NS Ghezi S et al Association of Traffic-Related Air Pollution with Newborn's Anthropometric Indexes at Birth. Environ Res (2022) 204:112000. 10.1016/j.envres.2021.112000
31.
Lamichhane DK Ryu J Leem JH Ha M Hong YC Park H et al Air Pollution Exposure during Pregnancy and Ultrasound and Birth Measures of Fetal Growth: A Prospective Cohort Study in Korea. Sci Total Environ (2018) 619-620:834–41. 10.1016/j.scitotenv.2017.11.058
32.
Stieb DM Chen L Hystad P Beckerman BS Jerrett M Tjepkema M et al A National Study of the Association between Traffic-Related Air Pollution and Adverse Pregnancy Outcomes in Canada, 1999-2008. Environ Res (2016) 148:513–26. 10.1016/j.envres.2016.04.025
33.
Kannan S Misra DP Dvonch JT Krishnakumar A . Exposures to Airborne Particulate Matter and Adverse Perinatal Outcomes: a Biologically Plausible Mechanistic Framework for Exploring Potential Effect Modification by Nutrition. Environ Health Perspect (2006) 114(11):1636–42. 10.1289/ehp.9081
34.
Slama R Darrow L Parker J Woodruff TJ Strickland M Nieuwenhuijsen M et al Meeting Report: Atmospheric Pollution and Human Reproduction. Environ Health Perspect (2008) 116(6):791–8. 10.1289/ehp.11074
35.
Clemente DB Casas M Vilahur N Begiristain H Bustamante M Carsin AE et al Prenatal Ambient Air Pollution, Placental Mitochondrial DNA Content, and Birth Weight in the INMA (Spain) and ENVIRONAGE (Belgium) Birth Cohorts. Environ Health Perspect (2016) 124(5):659–65. 10.1289/ehp.1408981
36.
Janssen BG Munters E Pieters N Smeets K Cox B Cuypers A et al Placental Mitochondrial DNA Content and Particulate Air Pollution during In Utero Life. Environ Health Perspect (2012) 120(9):1346–52. 10.1289/ehp.1104458
37.
Chen RJ Zhao Z Sun Q Lin Z Zhao A Wang C et al Size-fractionated Particulate Air Pollution and Circulating Biomarkers of Inflammation, Coagulation, and Vasoconstriction in a Panel of Young Adults. Epidemiology (2015) 26(3):328–36. 10.1097/ede.0000000000000273
38.
Martens DS Cox B Janssen BG Clemente DBP Gasparrini A Vanpoucke C et al Prenatal Air Pollution and Newborns' Predisposition to Accelerated Biological Aging. JAMA Pediatr (2017) 171(12):1160–7. 10.1001/jamapediatrics.2017.3024
39.
Han Y Jiang P Dong T Ding X Chen T Villanger GD et al Maternal Air Pollution Exposure and Preterm Birth in Wuxi, China: Effect Modification by Maternal Age. Ecotoxicol Environ Saf (2018) 157:457–62. 10.1016/j.ecoenv.2018.04.002
40.
Li G Kong L Li Z Zhang L Fan L Zou L et al Prevalence of Macrosomia and its Risk Factors in china: a Multicentre Survey Based on Birth Data Involving 101, 723 Singleton Term Infants. Paediatr Perinat Epidemiol (2014) 28(4):345–50. 10.1111/ppe.12133
41.
Ota E Ganchimeg T Morisaki N Vogel JP Pileggi C Ortiz-Panozo E et al Risk Factors and Adverse Perinatal Outcomes Among Term and Preterm Infants Born Small-For-Gestational-Age: Secondary Analyses of the WHO Multi-Country Survey on Maternal and Newborn Health. PLoS One (2014) 9(8):e105155. 10.1371/journal.pone.0105155
42.
Prefumo F Bhide A Sairam S Penna L Hollis B Thilaganathan B . Effect of Parity on Second-Trimester Uterine Artery Doppler Flow Velocity and Waveforms. Ultrasound Obstet Gynecol (2004) 23(1):46–9. 10.1002/uog.908
43.
Wilsher S Allen WR . The Effects of Maternal Age and Parity on Placental and Fetal Development in the Mare. Equine Vet J (2003) 35(5):476–83. 10.2746/042516403775600550
44.
Fowden AL Sferruzzi-Perri AN Coan PM Constancia M Burton GJ . Placental Efficiency and Adaptation: Endocrine Regulation. J Physiol (2009) 587:3459–72. 10.1113/jphysiol.2009.173013
45.
Roland MC Friis CM Voldner N Godang K Bollerslev J Haugen G et al Fetal Growth versus Birthweight: the Role of Placenta versus Other Determinants. PLoS One (2012) 7(6):e39324. 10.1371/journal.pone.0039324
46.
Yang J Zhou M Ou CQ Yin P Li M Tong S et al Seasonal Variations of Temperature-Related Mortality burden from Cardiovascular Disease and Myocardial Infarction in China. Environ Pollut (2017) 224:400–6. 10.1016/j.envpol.2017.02.020
47.
Scholl TO Chen XH . Vitamin D Intake during Pregnancy: Association with Maternal Characteristics and Infant Birth Weight. Early Hum Dev (2009) 85(4):231–4. 10.1016/j.earlhumdev.2008.10.006
Summary
Keywords
cross-sectional study, Wuhan, air pollution score, small for gestational age infant, joint association
Citation
Zhang F, Zhang X, Zhong Y, Zhu S, Zhao G, Zhang X, Li T, Zhang Y and Zhu W (2023) Joint Exposure to Ambient Air Pollutants Might Elevate the Risk of Small for Gestational Age (SGA) Infants in Wuhan: Evidence From a Cross-Sectional Study. Int J Public Health 67:1605391. doi: 10.3389/ijph.2022.1605391
Received
13 September 2022
Accepted
16 December 2022
Published
05 January 2023
Volume
67 - 2022
Edited by
Nino Kuenzli, Swiss Tropical and Public Health Institute (Swiss TPH), Switzerland
Reviewed by
Wojciech Hanke, Nofer Institute of Occupational Medicine, Poland
Updates
Copyright
© 2023 Zhang, Zhang, Zhong, Zhu, Zhao, Zhang, Li, Zhang and Zhu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhang, zhangyan_wch@163.com; Wei Zhu, weizhu@whu.edu.cn
†These authors have contributed equally to this work and share first authorship
This Original Article is part of the IJPH Special Issue Health in all Sustainable Development Goals
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.