- 1Respiratory Medicine Department of Xiangtan Central Hospital of Hunan Province, Xiangtan, Hunan, China
- 2Clinical Epidemiology Program, Ottawa Hospital Research Institute, Ottawa, ON, Canada
- 3Department of Pulmonary and Critical Care Medicine of Yuxi People’s Hospital of Yunnan Province, Yuxi, Yunnan, China
- 4School of Epidemiology and Public Health, University of Ottawa Faculty of Medicine, Ottawa, ON, Canada
- 5BORN (Better Outcome Registry Network) Ontario, Children’s Hospital of Eastern Ontario, Ottawa, ON, Canada
- 6Division of Respiratory, The Ottawa Hospital, Ottawa, ON, Canada
- 7Department of Obstetrics and Gynecology, University of Ottawa, Ottawa, ON, Canada
Objectives: Women’s health status is better than men but the opposite is true for female smokers who usually have poorer long-health outcomes than male smokers. The objectives of this study were to thoroughly reviewed and analyzed relevant literature and to propose a hypothesis that may explain this paradox phenomenon.
Methods: We conducted a search of literature from three English databases (EMBASE, MEDLINE, and Google Scholar) from inception to 13 November 2023. A combination of key words and/or subject headings in English was applied, including relevant terms for cigarette smoking, sex/gender, pregnancy, and health indicators. We then performed analysis of the searched literature.
Results: Based on this review/analysis of literature, we proposed a hypothesis that may explain this paradox phenomenon: female smokers have worse long-term health outcomes than male smokers because some of them smoke during pregnancy, and the adverse effects of cigarette smoking during pregnancy is much stronger than cigarette smoking during non-pregnancy periods.
Conclusion: Approval of our pregnancy-amplification theory could provide additional evidence on the adverse effect on women’s long-term health outcomes for cigarette smoking during pregnancy.
Introduction
Tobacco use is one of the most important risk factors for chronic diseases [1]. Globally, tobacco use accounted for about 7.69 million deaths and 200 million disability-adjusted life-years in 2019 alone [1]. On the other hand, tobacco use is a modifiable risk factor: quitting smoking will remove this risk. Tireless effort in the past decades has achieved some progresses in smoking cessation: prevalence of smoking had decreased among both males (27.5% reduction) and females (37.7% reduction) aged 15 years and older [1]. However, population growth has led to a significant increase in the total number of smokers: from 0.99 billion in 1990 to 1.14 billion in 2019 in the world [1]. Without continued effort in smoking cessation campaign, the annual toll of 7.69 million deaths and 200 million disability-adjusted life-years attributable to smoking will increase over the coming decades [1].
Health outcomes in female smokers are usually poorer than male smokers [2–4]. This is ironic as in general women have better health profile than men. According to the World Health Statistics 2018, women live longer than men in all regions, and the sex-gap in life expectancy was 4.3 years in 2000 and remained almost the same in 2016 (4.4 years) [5]. In this paper, we summarized studies comparing health outcomes between female smokers and male smokers, proposed study designs that could help explain this paradox phenomenon and discussed implications of the proposed studies.
Methods
We conducted a search of literature from three English databases (EMBASE, MEDLINE, and Google Scholar) from inception to 13 November 2023. A combination of key words and/or subject headings in English was applied, including relevant terms for cigarette, tobacco, smoking, sex, gender, pregnancy, gestation, health, life expectancy, cardiovascular diseases, diabetes, and cancers. Articles that did not include health outcomes data for both sexes were excluded in the comparison of health status between males and females. Articles that did not include smoking and health outcomes for both sexes were excluded in the comparison of health outcomes between male smokers and female smokers. In addition, we searched for eligible studies through the reference lists of identified studies. Because of the broadness of the issues covered in this review, no attempt for a systematic review was made. As a result, the search is not considered exhaustive. Most of the included studies have addressed well-known facts such as the sex-gap in life-expectancy/general health status and impacts of pregnancy on women’s organ systems. For less certain topics such as sex-differences in smoking effects, we have tried to be neutral and have included reports for or against, if such a literature was identified in the search.
All the identified papers were analyzed by the research team and were organized into the following sections: sex-differences in general health status, differences in health effect between female smokers and male smokers, and impact of pregnancy on long-term health of cigarette smoking. For studies that were considered essential for analysis, namely differences in health effect between female smokers and male smokers, quality of the original studies was assessed using Newcastle-Ottawa Scale [6]. To help illustrate our points, we created hypothesized scenarios of the male-female differences in effects of smoking with cardiovascular disease risk (expressed as relative risks). In all these scenarios, we used never smoking as the reference and compared with different smoking status for males and females.
Results
Comparison of General Health Status Between Women and Men
It has been consistently demonstrated that women have superior health status than men. We summarized the recent worldwide statistics in Table 1 which showed that females have longer life-expectancy than males [5]. In 2000, worldwide life-expectancy in females was 4.3 years higher than males and in 2016 the difference was 4.4 years [5]. The female superiority in health has been observed in other health outcomes as well [7–16]. For example, studies found that female patients with chronic heart failure had better survival than male patients [11]. An analysis of 8,630 patients with first myocardial infarction event in Northern Sweden found a higher long-term survival rate in women than men [12]. A Taiwan population-based study showed that the 5-year overall and cancer-specific survivals of colorectal cancer were significantly higher in female patients than in male patients [14]. Chatkin et al. observed that in 253 patients with non-small cell lung cancer who had undergone lung resections, the 5-year survival rate for women (85.5%) was much higher than for men (46.6%) [16]. North and Christiani found that female lung cancer patients experienced higher survival than male patients, regardless of stage, histology, or treatment modality [16]. Overall, these observations suggest that regardless of diseases, clinical manifestations, and treatments, female patients in general experienced superior progresses and survival.
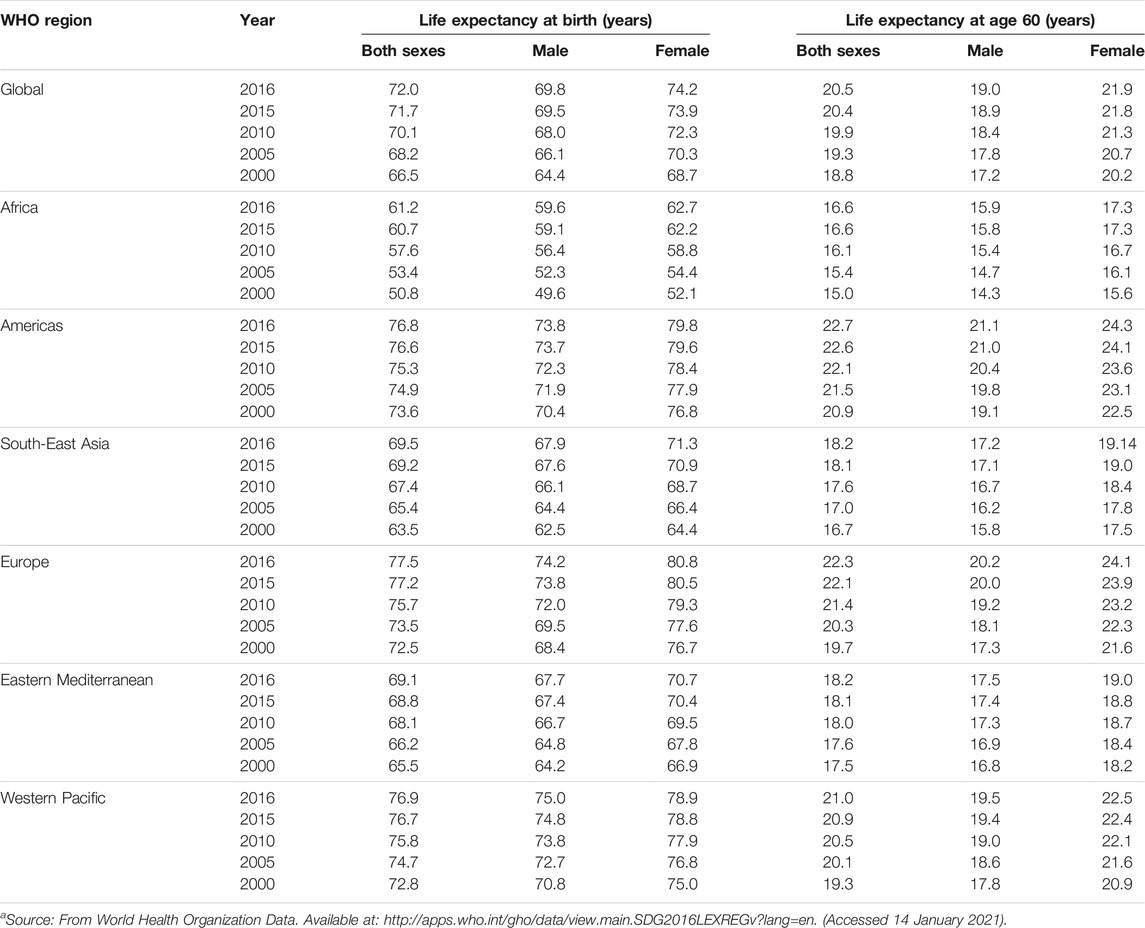
TABLE 1. Worldwide statistics on sex-specific life expectancy (Canada, 2023)a.
Comparison of Health Outcomes Between Female Smokers and Male Smokers
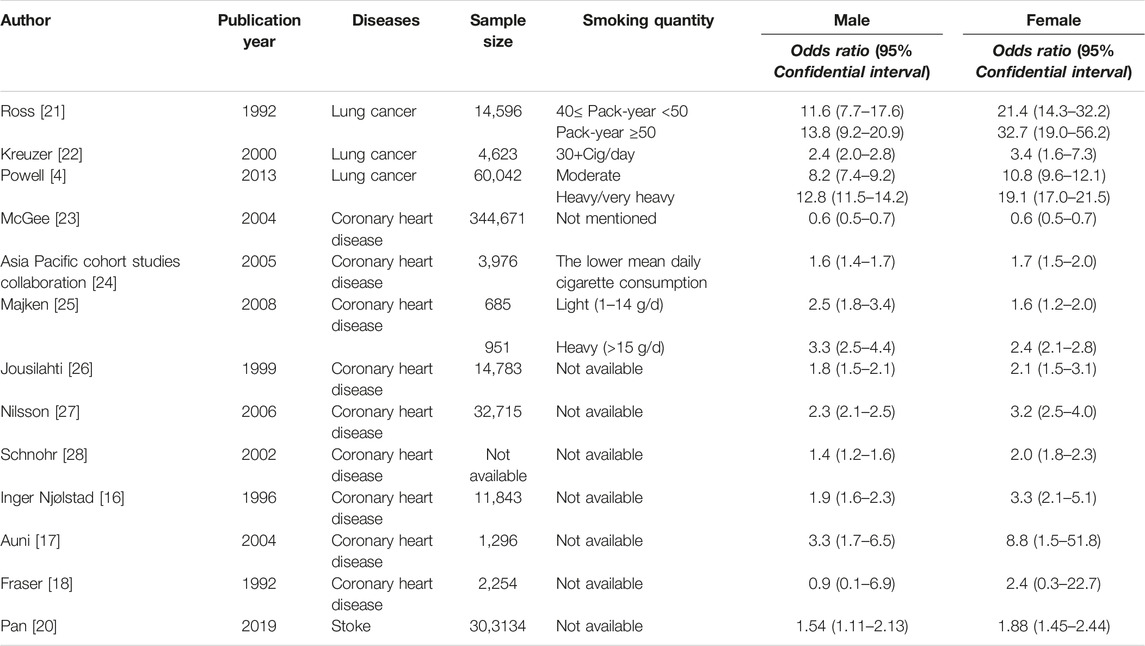
Contrary to the situation in the general population, female smokers were usually at greater risk for smoking-related chronic diseases than male smokers [2–4, 17–40]. We summarized recent studies that provided numerical comparison on the effects of cigarette smoking on chronic diseases between women and men (Table 2), which showed that in general, female smokers were at greater risk for smoking-related chronic diseases than male smokers. All the included studies were cohort or case-controls studies, with fairly good quality (Table 3). Powell et al observed that moderate/heavy female smokers were at higher risk for lung cancer than male smokers with the same amount of smoking [4]. This sex-difference in effects of cigarette smoking was particularly prominent for squamous and small cell lung cancers [31–34]. Female smokers had an increased risk for advanced colorectal neoplasia than male smokers [35]. In a longitudinal population study in UK, Prescott et al found that females who smoked cigarette had approximately a 50% increased risk of dying from vascular diseases than males who smoked cigarette [2]. In a systematic review, Mongraw-Chaffin et al summarized studies comparing the effect of cigarette smoking on coronary heart disease between males and females and found that in average female smokers had a 25% greater risk of coronary heart disease than male smokers [36]. Jamee et al made the same observation in a study in Gaza-Palestine [37]. Female smokers were more likely to experience cardiovascular complications than male smokers [38]. Another meta-analysis of the association between cigarette smoking and stroke found that current smokers had an increased risk of stroke compared with non-smokers (Odds ration (OR): 1.46, 95% CI: 1.04–2.07, p < .023), which was influenced by sex (OR in men 1.54 and OR in women 1.88) [21]. Female smokers with chronic obstructive pulmonary disease (COPD) were easier to develop airway obstruction after fewer numbers of cigarettes per lifetime than male smokers, and women with severe COPD have a 50% increased risk of death compared to men [39].
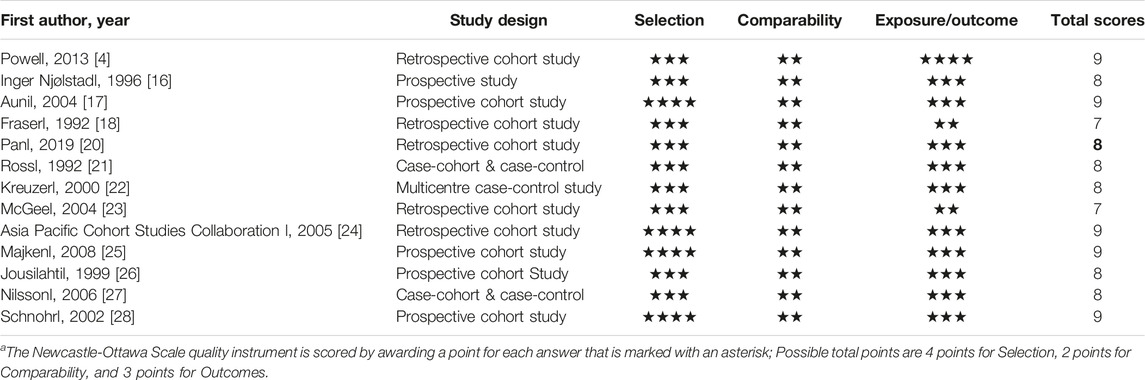
TABLE 3. Quality assessment of the included studies in Table 2 by Newcastle-Ottawa Scale for assessing the quality of the included studies (Canada, 2023)a.
Different from its harmful effect on most chronic diseases, cigarette smoking had a protective effect on Parkinson’s disease [40]. A meta-analysis of the association between cigarette smoking and Parkinson’s disease found that the protective effect of smoking against Parkinson’s disease was greater in male smokers than in female smokers [40]. We do not know the mechanisms of the protective effect of cigarette smoking on Parkinson’s disease. Regardless of the mechanisms, however, the results from a single smoking-disease association could not alter the hypothesis we have proposed in this paper that was based on majority of the relevant studies that we could identify.
Physiological Changes During Pregnancy and Its Potential Role on Long-Term Health Effects Among Female Smokers
Most women will go through pregnancy at some point in their life, and the female body undergoes tremendous anatomical and physiological changes, such as changes in cardiovascular, respiratory, endocrine, haematologic, renal, and gastrointestinal systems to accommodate the metabolic and physical demands of pregnancy [41–60]. Blood volume in pregnant women increases 30%–50% [41, 44]. Heart-beat rate increases by 15–20 beats per minute [40, 45] and cardiac output increases up to 30%–50% [41, 45] during pregnancy. The pH and PaO2 in the arterial blood gas during pregnancy are higher than nonpregnancy period while the PaCO2 are lower in pregnancy [52]. Alternations in hormone levels during pregnancy could induce postpartum anxiety [53–57]. The expanding of uterus during pregnancy could displace the digestive organs [41]. β-cells could be dilated in order to meet the metabolic demands of pregnancy [59]. Pregnancy could also trigger some diseases such as asthma attack [59].
Although many of the changes during pregnancy will gradually return to normal levels after childbirth, some changes, especially pathophysiological ones, are likely to be lifelong [61–63]. These changes could lead to increased absorption and circulation of the toxic substances contained in cigarette smoking, and therefore amplify the harm of cigarette smoking for female smokers, increasing the risks of both short-term pregnancy complications and long-term health outcomes. Previous studies have found associations of pregnancy complications with long-term health outcomes. In a study that followed 12,849 women affected by preeclampsia and 284,188 women without, van Walraven et al found that venous thromboembolism was more common in the preeclampsia group (0.12%, 41.7 events per 100 000 person years observation) than in any of the control groups (range 0.01%–0.08%, rates of 3.0–33.8 events per 100,000 person years observation) [61]. Based on a follow up study data, Smith et al estimated that a total of 18.2% of preeclamptic women and 1.7% of control women had a high 10-year risk, 31.3% of preeclamptic women and 5.1% of control women had a high 30-year risk, and 41.4% of preeclamptic women and 17.8% of control women had a high lifetime risk for cardiovascular disease [62]. In a longitudinal follow up of a large group of pregnant women (including both with or without gestational diabetes), Retnakaran & Shah found that each 1 mmol/L increment in the glucose challenge test result was associated with a 13% higher risk of cardiovascular disease after adjustment for age, ethnicity, income, and rurality [63]. Although direct evidence from human study on how pregnancy amplification effects for cigarette smoking is not available, animal disease models on metabolisms in pregnancy [64, 65] suggest such a possibility.
How to Explain the Differences of Health Effects Between Male Smokers and Female Smokers?
Cigarette smoking is causally responsible for many chronic diseases [66]. Around 140,000 premature deaths from cardiovascular diseases were caused by smoking in the United States each year [67]. Some researchers observed that ischemic heart disease incidence rises with increased dose of cigarette smoking [67, 68]. Nicotine could cross the placenta and concentrate in fetal blood and amniotic fluid in pregnant women who smoke cigarettes during pregnancy [69].
Smoking during pregnancy is an important risk factor for adverse outcomes for pregnant mothers and neonates [70–82]. Maternal smoking has also been linked to attention-deficit hyperactivity disorder, learning disabilities, behavioral problems, and increased the risk of nicotine addiction in the offspring [83–87]. Long term effects of maternal smoking on the offspring include obesity, type 2 diabetes, hypertension, asthma, COPD, childhood cancers, and reduced fertility in the offspring [87–92]. However, there are limited studies on the association between smoking during pregnancy and the long-term outcomes in women themselves.
Reasons behind sex differences in the effects of cigarette smoking are not clearly understood. Some investigators suggested that male-female differences in genetic susceptibility, hormones, and exposure to other environmental factors may explain the male-female differences in health hazards of cigarette smoking. We hypothesize that female smokers have worse long-term health outcomes than male smokers because some of them smoke during pregnancy, and the adverse effect of cigarette smoking during pregnancy is much stronger than the effect of cigarette smoking during non-pregnancy period. The graphic presentation of the potential mechanisms that may explain why female smokers have poorer long-term health outcomes as compared with male smokers is displayed in Figure 1.
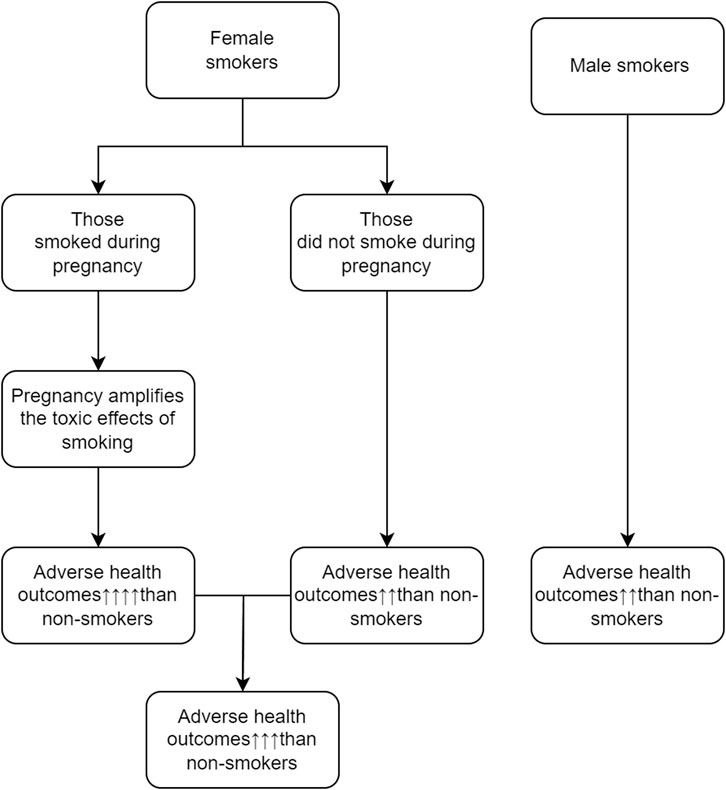
FIGURE 1. Potential mechanisms that may explain why female smokers have poorer long-term health outcomes (Canada, 2023).
Although an association between maternal cigarette smoking and decreased risk of preeclampsia and gestational hypertension has been observed [93, 94], this did not provide evidence against our proposal. Preeclampsia/gestational hypertension is a heterogeneous entity, with severe preeclampsia/gestational hypertension carries short- and long-term consequences to the mother and their children, mild preeclampsia/gestational hypertension does not seem affect the health of the mother and their children [94]. Maternal cigarette smoking, while reduce the incidence of preeclampsia/gestational hypertension, mortality and morbidity in smokers who developed preeclampsia/gestational hypertension during pregnancy were higher in non-smokers who developed preeclampsia/gestational hypertension during pregnancy [95–97]. This observation suggests that cigarette smoking may reduce the incidence of mild form of preeclampsia/gestational hypertension but may increase the severity of preeclampsia/gestational hypertension [95–97]. Because long-term health outcomes are more likely associated with severe form of preeclampsia/gestational hypertension [62], maternal cigarette smoking carries long-term harm to them.
Hypotheses other than pregnancy on the higher risk of cigarette smoking in female smokers than in male smokers, including differences in genetics, hormones, environmental exposures (including passive smoking), and difficulties in quitting smoking have been proposed [98–103]. Possible interaction between smoking and hormonal factors may need to be considered in the observed male-female difference in smoking effects. Elevated arginine vasopressin level has been associated with chronic diseases including cardiovascular diseases [99]. In a small study, Guaderrama at al observed that female smokers had higher arginine vasopressin level than male smokers [99]. In a study in women undergoing in-vitro fertilization treatment, active smokers (defined as smoking at least one cigarette per day at the time of procedure) had significantly lower anti-mullerian hormone levels and antral follicle counts, and worse overall in-vitro fertilization outcomes than non-smokers (defined as never smoke or who quitted smoke for 1 year prior to the treatment) [100, 101]. Bennett et al [102] reported that for never-smoking women, passive smoking was responsible for between 42% and 49% of the lung cancer cases. However, this type of study could not help to explain the difference of health outcomes in male smokers versus female smokers. In a meta-analysis of the 14 placebo-controlled nicotine patch trials (N = 6,250) for which long-term (6 months) clinical outcome results could be determined separately by sex, women had greater difficulty quitting smoking [103]. The reasons why female smokers are more difficult to quit smoking remains unknown, however.
Previous Studies Comparing Female and Male Differences in Health Effect of Cigarette Smoking
Previous studies comparing differences in the effects of cigarette smoking between female smokers and male smokers are limited by the lack of an appropriate control group to test a hypothesis. A direct comparison between male smokers with female smokers can reveal the sex difference in health effect of cigarette smoking. However, there are major differences in anatomy, physiology, genetics, and exposure to varies environmental factors which may confound the association of smoking with sex. All these differences could explain the smoking effects, but could not test a hypothesis why female smokers have poorer long-term health outcomes on specific and unique female factors.
The sex differences in anatomy, physiology, genetics, and exposure to environmental factors, on the other hand, are usually not modifiable, so a study solely comparing outcomes between male and female smokers may have limited implication in terms of public health and disease prevention. A comparison of long-term health outcomes between female smokers who are childbearing and who are childless may be interesting. However, very rare for a study examined the rate of cigarette smoking and its association with long-term outcomes in childless women specifically. We made specific search of literature on this topic and could not identify a published study that compared long-term health outcomes between female smokers who are childbearing and those who are childless.
Proposed Studies to Test the Hypothesis of Pregnancy Amplifies the Long-Term Harm of Cigarette Smoking in Female Smokers
Our hypothesis that pregnancy amplifies the consequence of cigarette smoking in female smokers, if proven true, has major implication in prevention, as it emphasizes the importance of smoking cessation during pregnancy in terms of both short- and long-term health outcomes.
It is rather difficult to test the hypothesis that effect of tobacco smoking during pregnancy is stronger than tobacco smoking during non-pregnancy periods directly, because of the difficulties to separate the person-time from the same woman during pregnancy period versus non-pregnancy period and relate that to outcome. As a result, we propose a simple approach by comparing the effects of female smokers versus male smokers: overall female smokers, restricting to female smokers who quitted smoking during pregnancy, and restricting to female smokers who were smoking free during pregnancy and thereafter. We created hypothetical scenarios to illustrate our points, using cardiovascular disease as an example (Table 4). In this example, under the ideal scenario (female smokers who quitted smoking in pregnancy entirely and maintained smoke free postpartum entirely), the effect of tobacco smoking in female smokers on cardiovascular disease (1.25) should be smaller than male smokers (1.75), consisting with the better general health status in females than in males.

TABLE 4. Effects of tobacco smoking on cardiovascular disease between female smokers and male smokers under different hypothetic scenarios (Canada, 2023)a.
Another method to test this hypothesis is to compare long-term health outcomes between female smokers who had children and those who were childless. If the outcomes are poorer for female smokers who were childbearing as compare with female smokers who were childless, our hypothesis should be valid as for women who were childless all smoking were non-pregnancy while for women who were childbearing at least part of smoking were pregnancy.
Discussion
Changing human behaviours is one of the most difficult tasks for public health effort. Despite many studies showings the harmful effects of cigarette smoking, about 10%–17% of women smoked during pregnancy worldwide [104–109]. Of those smoked during pregnancy, 40% quitted at the first trimester of pregnancy. However, about 60% of those who quitted smoking in pregnancy resumed smoking 6 months postpartum [108, 109]. These statistics indicate the challenges we are facing and that more evidence from solid studies is needed, including the ones proposed in this paper.
Limitations of our analysis should be acknowledged. First, because of the scope and breadth of the issues addressed in this paper, we have not attempted to conduct a systematic review. The lack of systematic review may result in biased assessment of the adverse health effect between female smokers and male smokers and between pregnancy smoking and non-pregnancy smoking. Systematic review is still needed to resolve this paradox phenomenon in health effects of smoking. Second, because of the limited number of identified relevant original studies and because of major heterogeneity of the included studies, we have not been able to summarize the evidence by a meta-analysis. The inability to make a meta-analysis for the included original studies made it necessary in some degree of arbitrary in analysis and cautions should apply in generalizing the conclusions based on this type of analysis. Third, no data from childless women was available, so we could not make a direct comparison of the health effect between smoking during pregnancy versus smoking in no pregnant women. Fourth, because of the unavailability in original studies, in the comparison of general health status between males and females, smokers were not excluded. Fifth, the theory that pregnancy amplifies the harmful effects of cigarette smoking was inferred indirectly from physiological changes during pregnancy and from animal experiments, not from direct human studies. Finally, sex differences in socio-economic status, access to healthcare, and rates of smoking, alcohol consumption, and substances uses, were not considered in the comparison of general health status between men and women. Major sex differences, especially in terms of higher prevalence on smoking, may explain some of the poorer general health status in men.
In conclusion, based on thorough search and analysis of relevant literature, we provide a hypothesis in this paper that may explain the difference between the effect of cigarette smoking on long-term health outcomes of male and female smokers: pregnancy amplifies the effects of tobacco smoking, leading to higher risk of adverse long-term health outcomes in female smokers than in male smokers. This hypothesis could be tested by study designs proposed in this paper. Our hypothesis that pregnancy amplifies the consequence of cigarette smoking in female smokers, if proven true, may help in campaigns aiming at emphasizing the importance of quitting cigarette smoking during pregnancy and postpartum. Large scale, longitudinal studies in diverse populations with the design proposed by this paper would be particularly valuable.
Author Contributions
SP and SW developed the concept and supervised the study. LY and YZ searched literature, performed data analysis, and drafted the manuscript. MJ, WW, and YG conducted additional search of literature and data analysis. SW and SP made critical appraisal and revised the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was supported in part by Canadian Institutes for Health Research (FND-148438) and Natural Science Foundation of Hunan Province of China [No. 2 (2022) 2022JJ80031].
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Abbreviations
CI, Confidence interval; CHD, Coronary Heart Disease; COPD, Chronic obstructive pulmonary disease; OR, Odds ratio; PaCO2, Partial Pressure of Carbon Dioxide; PaO2, Partial Pressure of Dioxide.
References
1. GBD 2019 Tobacco Collaborators. Spatial, Temporal, and Demographic Patterns in Prevalence of Smoking Tobacco Use and Attributable Disease Burden in 204 Countries and Territories, 1990-2019: A Systematic Analysis From the Global Burden of Disease Study 2019. Lancet (2021) 397:2337–60. doi:10.1016/S0140-6736(21)01169-7
2. Prescott, E, Hippe, M, Schnohr, P, Hein, HO, and Vestbo, J. Smoking and Risk of Myocardial Infarction in Women and Men: Longitudinal Population Study. Br Med J (1998) 316:1043–7. doi:10.1136/bmj.316.7137.1043
3. Han, MLK, Postma, D, Mannino, DM, Giardino, ND, Buist, S, Curtis, JL, et al. Gender and Chronic Obstructive Pulmonary Disease: Why It Matters. Am J Respir Crit Care Med (2007) 176:1179–84. doi:10.1164/rccm.200704-553CC
4. Powell, HA, Iyen-omofoman, B, Hubbard, RB, Baldwin, DR, and Tata, LJ. The Association Between Smoking Quantity and Lung Cancer in Men and Women. Chest (2013) 143:123–9. doi:10.1378/chest.12-1068
5. Ali, S, Rose Alinda, A, and Syed Norris, H. World Health Statistics 2018: Monitoring Health for the Sustainable Development Goals. Geneva: World Health Organization (2018).
6. Wells, GA, Shea, B, Connell, DO, Peterson, J, Welch, V, Losos, M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses (2021). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed November 15, 2023).
7. Kontis, V, Bennett, JE, Mathers, CD, Li, G, Foreman, K, and Ezzati, M. Future Life Expectancy in 35 Industrialised Countries: Projections With a Bayesian Model Ensemble. Lancet (2017) 389(10076):1323–35. doi:10.1016/S0140-6736(16)32381-9
8. Tokudome, S, Hashimoto, S, and Igata, A. Life Expectancy and Healthy Life Expectancy of Japan: The Fastest Graying Society in the World. BMC Res Notes (2016) 9(1):482–6. doi:10.1186/s13104-016-2281-2
9. Storeng, SH, Krokstad, S, Westin, S, and Sund, ER. Decennial Trends and Inequalities in Healthy Life Expectancy: The HUNT Study, Norway. Scand J Public Health (2018) 46(1):124–31. doi:10.1177/1403494817695911
10. Sundberg, L, Agahi, N, Fritzell, J, and Fors, S. Why Is the Gender Gap in Life Expectancy Decreasing? The Impact of Age- and Cause-Specific Mortality in Sweden 1997–2014. Int J Public Health (2018) 63(6):673–81. doi:10.1007/s00038-018-1097-3
11. Sakata, Y, Miyata, S, Nochioka, K, Miura, M, Takada, T, Tadaki, S, et al. Gender Differences in Clinical Characteristics, Treatment and Long-Term Outcome in Patients With Stage C/D Heart Failure in Japan: Report From the Chart-2 Study. Circ J (2014) 78(2):428–35. doi:10.1253/circj.CJ-13-1009
12. Isaksson, RM, Jansson, JH, Lundblad, D, Näslund, U, Zingmark, K, and Eliasson, M. Better Long-Term Survival in Young and Middle-Aged Women Than in Men After a First Myocardial Infarction Between 1985 and 2006. An Analysis of 8630 Patients in the Northern Sweden MONICA Study. BMC Cardiovasc Disord (2011) 11(1):1. doi:10.1186/1471-2261-11-1
13. Sandler, BJ, Wang, Z, Hancock, JG, Boffa, DJ, Detterbeck, FC, and Kim, AW. Gender, Age, and Comorbidity Status Predict Improved Survival With Adjuvant Chemotherapy Following Lobectomy for Non-Small Cell Lung Cancers Larger Than 4 Cm. Ann Surg Oncol (2016) 23(2):638–45. doi:10.1245/s10434-015-4902-8
14. Chou, CL, Weng, SF, Lin, JK, and Chang, SC. Role for Gender in Colorectal Cancer Risk: A Taiwan Population-Based Study. Int J Colorectal Dis (2013) 28(7):1001–8. doi:10.1007/s00384-013-1647-3
15. Chatkin, JM, Abreu, CM, Fritscher, CC, Wagner, MB, and Pinto, JALF. Is There a Gender Difference in Non-Small Cell Lung Cancer Survival? Gend Med (2004) 1(1):41–7. doi:10.1016/S1550-8579(04)80009-3
16. North, CM, and Christiani, DC. Women and Lung Cancer: What Is New? Semin Thorac Cardiovasc Surg (2013) 25(2):87–94. doi:10.1053/j.semtcvs.2013.05.002
17. Njølstad, I, Arnesen, E, and Lund-Larsen, PG. Smoking, Serum Lipids, Blood Pressure, and Sex Differences in Myocardial Infarction. A 12-Year Follow-Up of the Finnmark Study. Circulation (1996) 93:450–6. doi:10.1161/01.cir.93.3.450
18. Auni, J, Saara, K, Seppo, L, Tapani, R, Kalevi, P, and Markku, L. Gender Difference in the Impact of Type 2 Diabetes on Coronary Heart Disease Risk. Diabetes Care (2004) 27(12):2898–904. doi:10.2337/diacare.27.12.2898
19. Fraser, GE, Strahan, TM, Sabaté, J, Beeson, WL, and Kissinger, D. Effects of Traditional Coronary Risk Factors on Rates of Incident Coronary Events in a Low-Risk Population: The Adventist Health Study. Circulation (1992) 86(2):406–13. doi:10.1161/01.CIR.86.2.406
20. Prescott, E, Osler, M, Andersen, PK, Hein, HO, Borch-Johnsen, K, Lange, P, et al. Mortality in Women and Men in Relation to Smoking. Int J Epidemiol (1998) 27(1):27–32. doi:10.1093/ije/27.1.27
21. Pan, B, Jin, X, Jun, L, Qiu, S, Zheng, Q, and Pan, M. The Relationship Between Smoking and Stroke: A Meta-Analysis. Medicine (Baltimore) (2019) 98(12):e14872. doi:10.1097/MD.0000000000014872
22. Brownson, RC, Chang, JC, and Davis, JR. Gender and Histologic Type Variations in Smoking-Related Risk of Lung Cancer. Epidemiology (1992) 3(1):61–4. doi:10.1097/00001648-199201000-00012
23. Kreuzer, M, Boffetta, P, Whitley, E, Ahrens, W, Gaborieau, V, Heinrich, J, et al. Gender Differences in Lung Cancer Risk by Smoking: A Multicentre Case-Control Study in Germany and Italy. Br J Cancer (2000) 82:227–33. doi:10.1054/bjoc.1999.0904
24. McGee, DL. Smoking, Body Weight, and CHD Mortality in Diverse Populations. Prev Med (Baltim) (2004) 38(6):834–40. doi:10.1016/j.ypmed.2003.12.022
25. Asia Pacific Cohort Studies Collaboration, Lam, TH, Barzi, F, Patel, A, Gu, D, Rodgers, A, et al. Smoking, Quitting, and the Risk of Cardiovascular Disease Among Women and Men in the Asia-Pacific Region. Int J Epidemiol (2005) 34(5):1036–45. doi:10.1093/ije/dyi104
26. Jensen, MK, Chiuve, SE, Rimm, EB, Dethlefsen, C, Tjønneland, A, Joensen, AM, et al. Obesity, Behavioral Lifestyle Factors, and Risk of Acute Coronary Events. Circulation (2008) 117(24):3062–9. doi:10.1161/CIRCULATIONAHA.107.759951
27. Jousilahti, P, Vartiainen, E, Tuomilehto, J, and Puska, P. Sex, Age, Cardiovascular Risk Factors, and Coronary Heart Disease: A Prospective Follow-Up Study of 14 786 Middle-Aged Men and Women in Finland. Circulation (1999) 99(9):1165–72. doi:10.1161/01.cir.99.9.1165
28. Nilsson, PM, Nilsson, JÅ, and Berglund, G. Population-Attributable Risk of Coronary Heart Disease Risk Factors During Long-Term Follow-Up: The Malmö Preventive Project. J Intern Med (2006) 260(2):134–41. doi:10.1111/j.1365-2796.2006.01671.x
29. Schnohr, P, Jensen, JS, Scharling, H, and Nordestgaard, BG. Coronary Heart Disease Risk Factors Ranked by Importance for the Individual and Community: A 21 Year Follow-Up of 12 000 Men and Women From the Copenhagen City Heart Study. Eur Heart J (2002) 23(8):620–6. doi:10.1053/euhj.2001.2842
30. Xu, X, Li, B, and Wang, L. Gender Difference in Smoking Effects on Adult Pulmonary Function. Eur Respir J (1994) 7(3):477–83. doi:10.1183/09031936.94.07030477
31. Brownson, RC, Chang, JC, DavisBrownson, JC, Chang, JC, and Davis, JR. Gender and Histologic Type Variations in Smoking-Related Risk of Lung Cancer. Epidemiology (1992) 3(1):61–4. doi:10.1097/00001648-199201000-00012
32. Harris, RE, Zang, EA, Anderson, JI, and Wynder, EL. Race and Sex Differences in Lung Cancer Risk Associated With Cigarette Smoking. Int J Epidemiol (1993) 22(4):592–9. doi:10.1093/ije/22.4.592
33. Risch, HA, Howe, GR, Jain, M, Burch, JD, Holowaty, EJMA, and Miller, AB. Are Female Smokers at Higher Risk for Lung Cancer Than Male Smokers? A Case-Control Analysis by Histologic Type. Am J Epidemiol (1993) 138:281–93. doi:10.1093/oxfordjournals.aje.a116857
34. Zang, EA, and Wynder, EL. Differences in Lung Cancer Risk Between Men and Women: Examination of the Evidence. J Natl Cancer Inst (1996) 88(3):183–92. doi:10.1093/jnci/88.3-4.183
35. Anderson, JC, Moezardalan, K, Messina, CR, Latreille, M, and Shaw, RD. Smoking and the Association of Advanced Colorectal Neoplasia in an Asymptomatic Average Risk Population: Analysis of Exposure and Anatomical Location in Men and Women. Dig Dis Sci (2011) 56(12):3616–23. doi:10.1007/s10620-011-1814-8
36. Mongraw-Chaffin, ML, Peters, SAE, Huxley, RR, and Woodward, M. The Sex-Specific Association Between BMI and Coronary Heart Disease: A Systematic Review and Meta-Analysis of 95 Cohorts With 1·2 Million Participants. Lancet Diabetes Endocrinol (2015) 3(6):437–49. doi:10.1016/S2213-8587(15)00086-8
37. Jamee, A, Abed, Y, and Jalambo, MO. Gender Difference and Characteristics Attributed to Coronary Artery Disease in Gaza-Palestine. Glob J Health Sci (2013) 5(5):51–6. doi:10.5539/gjhs.v5n5p51
38. Howe, M, Leidal, A, Montgomery, D, and Jackson, E. Role of Cigarette Smoking and Gender in Acute Coronary Syndrome Events. Am J Cardiol (2011) 108(10):1382–6. doi:10.1016/j.amjcard.2011.06.059
39. Han, MLK, Postma, D, Mannino, DM, Giardino, ND, Buist, S, Curtis, JL, et al. Gender and Chronic Obstructive Pulmonary Disease: Why It Matters. Am J Respir Crit Care Med (2007) 176(12):1179–84. doi:10.1164/rccm.200704-553CC
40. Li, X, Li, W, Liu, G, Shen, X, and Tang, Y. Association Between Cigarette Smoking and Parkinson’s Disease: A Meta-Analysis. Arch Gerontol Geriatr (2015) 61(3):510–6. doi:10.1016/j.archger.2015.08.004
41. Tan, EK, and Tan, EL. Alterations in Physiology and Anatomy During Pregnancy. Best Pract Res Clin Obstet Gynaecol (2013) 27(6):791–802. doi:10.1016/j.bpobgyn.2013.08.001
42. Hegewald, MJ, and Crapo, RO. Respiratory Physiology in Pregnancy. Clin Chest Med (2011) 32(1):1–13. doi:10.1016/j.ccm.2010.11.001
43. Contreras, G, GutiéRrez, M, Beroíza, T, Fantín, A, Oddó, H, Villarroel, L, et al. Ventilatory Drive and Respiratory Muscle Function in Pregnancy. Am Rev Respir Dis (1991) 144(4):837–41. doi:10.1164/ajrccm/144.4.837
44. Bernstein, IM, Ziegler, W, and Badger, GJ. Plasma Volume Expansion in Early Pregnancy. Obstet Gynecol (2001) 97(5):669–72. doi:10.1016/s0029-7844(00)01222-9
45. San-Frutos, L, Engels, V, Zapardiel, I, Perez-Medina, T, Almagro-Martinez, J, Fernandez, R, et al. Hemodynamic Changes During Pregnancy and Postpartum: A Prospective Study Using Thoracic Electrical Bioimpedance. J Matern Neonatal Med (2011) 24(11):1333–40. doi:10.3109/14767058.2011.556203
46. Ngene, NC, and Moodley, J. Physiology of Blood Pressure Relevant to Managing Hypertension in Pregnancy. J Matern Neonatal Med (2019) 32(8):1368–77. doi:10.1080/14767058.2017.1404569
47. Robson, SC, Dunlop, W, Moore, M, and Hunter, S. Haemodynamic Changes During the Puerperium: A Doppler and M-Mode Echocardiographic Study. BJOG (1987) 94(11):1028–39. doi:10.1111/j.1471-0528.1987.tb02286.x
48. Bobrowski, RA. Pulmonary Physiology in Pregnancy. Clin Obstet Gynecol (2010) 53(2):285–300. doi:10.1097/GRF.0b013e3181e04776
49. Ellegård, EK. Pregnancy Rhinitis. Immunol Allergy Clin North Am (2006) 26(1):119–35. vii. doi:10.1016/j.iac.2005.10.007
50. Toppozada, H, Michaels, L, Toppozada, M, El-Ghazzawl, I, Talaat, M, and Elwany, S. The Human Respiratory Nasal Mucosa in Pregnancy. An Electron Microscopic and Histochemical Study. J Laryngol Otol (1982) 96(7):613–26. doi:10.1017/S0022215100092902
51. Demir, UL, Demir, BC, Oztosun, E, Uyaniklar, OO, and Ocakoglu, G. The Effects of Pregnancy on Nasal Physiology. Int Forum Allergy Rhinol (2015) 5(2):162–6. doi:10.1002/alr.21438
52. Dinč, H, Esen, F, Demirci, A, Sari, A, and Resit Gümele, H. Pituitary Dimensions and Volume Measurements in Pregnancy and Post Partum. MR Assessment. Acta Radiol (2010) 39(1):64–9. doi:10.1080/02841859809172152
53. Fallon, V, Groves, R, Halford, JCG, Bennett, KM, and Harrold, JA. Postpartum Anxiety and Infant-Feeding Outcomes: A Systematic Review. J Hum Lact (2016) 32(4):740–58. doi:10.1177/0890334416662241
54. Glasheen, C, Richardson, GA, and Fabio, A. A Systematic Review of the Effects of Postnatal Maternal Anxiety on Children. Arch Womens Ment Health (2010) 13(1):61–74. doi:10.1007/s00737-009-0109-y
55. Wenzel, A, Haugen, EN, Jackson, LC, and Robinson, K. Prevalence of Generalized Anxiety at Eight Weeks Postpartum. Arch Womens Ment Health (2003) 6(1):43–9. doi:10.1007/s00737-002-0154-2
56. Paul, IM, Downs, DS, Schaefer, EW, Beiler, JS, and Weisman, CS. Postpartum Anxiety and Maternal-Infant Health Outcomes. Pediatrics (2013) 131(4):e1218–24. doi:10.1542/peds.2012-2147
57. Lonstein, JS. Regulation of Anxiety During the Postpartum Period. Front Neuroendocrinol (2007) 28(2-3):115–41. doi:10.1016/j.yfrne.2007.05.002
58. Dure-Smith, P. Pregnancy Dilatation of the Urinary Tract. The Iliac Sign and Its Significance. Radiology (1970) 96(3):545–50. doi:10.1148/96.3.545
59. Plows, J, Stanley, J, Baker, P, Reynolds, C, and Vickers, M. The Pathophysiology of Gestational Diabetes Mellitus. Int J Mol Sci (2018) 19(11):3342. doi:10.3390/ijms19113342
60. Baghlaf, H, Spence, AR, Czuzoj-Shulman, N, and Abenhaim, AH. Pregnancy Outcomes Among Women With Asthma. J Matern Fetal Neonatal Med (2019) 32(8):1325–31. doi:10.1080/14767058.2017.1404982
61. van Walraven, C, Mamdani, M, Katib, Y, Walker, MC, and Rodger, M. Risk of Subsequent Thromboembolism for Patients With Pre-Eclampsia. Br Med J (2003) 326:791–2. doi:10.1136/bmj.326.7393.791
62. Smith, GN, Pudwell, J, Walker, MC, and Wen, SW. Ten-Year, Thirty-Year, and Lifetime Cardiovascular Disease Risk Estimates Following a Pregnancy Complicated by Preeclampsia. J Obstet Gynaecol Can (2012) 34(9):830–5. doi:10.1016/S1701-2163(16)35381-6
63. Retnakaran, R, and Shah, BR. Glucose Screening in Pregnancy and Future Risk of Cardiovascular Disease in Women: A Retrospective, Population-Based Cohort Study. Lancet Diabetes Endocrinol (2019) 7:378–84. doi:10.1016/S2213-8587(19)30077-4
64. Sferruzzi-Perri, AN, Lopez-Tello, J, Napso, T, and Yong, HEJ. Exploring the Causes and Consequences of Maternal Metabolic Maladaptations During Pregnancy: Lessons From Animal Models. Placenta (2020) 98:43–51. doi:10.1016/j.placenta.2020.01.015
65. Morris, EA, Mandalà, M, Ko, NL, and Osol, G. Postpartum Persistence of Maternal Uterine Vascular Gestational Adaptation in Rodents. Reprod Sci (2020) 27(2):611–20. doi:10.1007/s43032-019-00062-z
66. Bartal, M. Health Effects of Tobacco Use and Exposure. Monaldi Arch Chest Dis - Pulm Ser (2001) 56(6):545–54.
67. Burns, DM. Epidemiology of Smoking-Induced Cardiovascular Disease. Prog Cardiovasc Dis (2003) 46(1):11–29. doi:10.1016/S0033-0620(03)00079-3
68. Law, MR, and Wald, NJ. Environmental Tobacco Smoke and Ischemic Heart Disease. Prog Cardiovasc Dis (2003) 46(1):31–8. doi:10.1016/S0033-0620(03)00078-1
69. Lambers, DS, and Clark, KE. The Maternal and Fetal Physiologic Effects of Nicotine. Semin Perinatol (1996) 20(2):115–26. doi:10.1016/S0146-0005(96)80079-6
70. Heffner, LJ, Sherman, CB, Speizer, FE, and Weiss, ST. Clinical and Environmental Predictors of Preterm Labor. Obstet Gynecol (1993) 81(5):750–7.
71. Haslinger, C, Bamert, H, Rauh, M, Burkhardt, T, and Schäffer, L. Effect of Maternal Smoking on Stress Physiology in Healthy Neonates. J Perinatol (2018) 38(2):132–6. doi:10.1038/jp.2017.172
72. DiFranza, JR, and Lew, RA. Effect of Maternal Cigarette Smoking on Pregnancy Complications and Sudden Infant Death Syndrome. J Fam Pract (1995) 40(4):117–94. doi:10.3109/13814789509160303
73. Salihu, HM, and Wilson, RE. Epidemiology of Prenatal Smoking and Perinatal Outcomes. Early Hum Dev (2007) 83(11):713–20. doi:10.1016/j.earlhumdev.2007.08.002
74. Al-Sahab, B, Saqib, M, Hauser, G, and Tamim, H. Prevalence of Smoking During Pregnancy and Associated Risk Factors Among Canadian Women: A National Survey. BMC Pregnancy Childbirth (2010) 10(1):24. doi:10.1186/1471-2393-10-24
75. Bachir, R, and Chaaya, M. Maternal Smoking: Determinants and Associated Morbidity in Two Areas in Lebanon. Matern Child Health J (2008) 12(3):298–307. doi:10.1007/s10995-007-0242-z
76. Adams, EK, Miller, VP, Ernst, C, Nishimura, BK, Melvin, C, and Merritt, R. Neonatal Health Care Costs Related to Smoking During Pregnancy. Health Econ (2002) 11(3):193–206. doi:10.1002/hec.660
77. Centers for Disease Control and Prevention (CDC). Smoking During Pregnancy-United States, 1990-2002. Morb Mortal Wkly Rep (2004) 53(2):911–5. doi:10.1097/00132582-200506000-00050
78. Kyrklund-Blomberg, NB, Granath, F, and Cnattingius, S. Maternal Smoking and Causes of Very Preterm Birth. Acta Obstet Gynecol Scand (2005) 84(6):572–7. doi:10.1111/j.0001-6349.2005.00848.x
79. Oyelese, Y, and Smulian, JC. Placenta Previa, Placenta Accreta, and Vasa Previa. Obstet Gynecol (2006) 107(4):927–41. doi:10.1097/01.AOG.0000207559.15715.98
80. Tikkanen, M, Nuutila, M, Hiilesmaa, V, Paavonen, J, and Ylikorkala, O. Prepregnancy Risk Factors for Placental Abruption. Acta Obstet Gynecol Scand (2006) 85(1):40–4. doi:10.1080/00016340500324241
81. Ng, SP, and Zelikoff, JT. Smoking During Pregnancy: Subsequent Effects on Offspring Immune Competence and Disease Vulnerability in Later Life. Reprod Toxicol (2007) 23(3):428–37. doi:10.1016/j.reprotox.2006.11.008
82. Kolås, T, Nakling, J, and Salvesen, KÅ. Smoking During Pregnancy Increases the Risk of Preterm Births Among Parous Women. Acta Obstet Gynecol Scand (2000) 79(8):644–8. doi:10.1034/j.1600-0412.2000.079008644.x
83. Rogers, JM. Tobacco and Pregnancy. Reprod Toxicol (2009) 28(2):152–60. doi:10.1016/j.reprotox.2009.03.012
84. Pauly, JR, and Slotkin, TA. Maternal Tobacco Smoking, Nicotine Replacement and Neurobehavioural Development. Acta Paediatr Int J Paediatr (2008) 97(10):1331–7. doi:10.1111/j.1651-2227.2008.00852.x
85. Dwyer, JB, McQuown, SC, and Leslie, FM. The Dynamic Effects of Nicotine on the Developing Brain. Pharmacol Ther (2009) 122(2):125–39. doi:10.1016/j.pharmthera.2009.02.003
86. Cornelius, MD, and Day, NL. Developmental Consequences of Prenatal Tobacco Exposure. Curr Opin Neurol (2009) 22(2):121–5. doi:10.1097/WCO.0b013e328326f6dc
87. Bruin, JE, Gerstein, HC, and Holloway, AC. Long-Term Consequences of Fetal and Neonatal Nicotine Exposure: A Critical Review. Toxicol Sci (2010) 116(2):364–74. doi:10.1093/toxsci/kfq103
88. Zheng, Y, Ritzenthaler, JD, Roman, J, and Han, SW. Nicotine Stimulates Human Lung Cancer Cell Growth by Inducing Fibronectin Expression. Am J Respir Cel Mol Biol (2007) 37(6):681–90. doi:10.1165/rcmb.2007-0051OC
89. Martin, JW, Mousa, SS, Shaker, O, and Mousa, SA. The Multiple Faces of Nicotine and Its Implications in Tissue and Wound Repair. Exp Dermatol (2009) 18(6):497–505. doi:10.1111/j.1600-0625.2009.00854.x
90. Catassi, A, Servent, D, Paleari, L, Cesario, A, and Russo, P. Multiple Roles of Nicotine on Cell Proliferation and Inhibition of Apoptosis: Implications on Lung Carcinogenesis. Mutat Res - Rev Mutat Res (2008) 659(3):221–31. doi:10.1016/j.mrrev.2008.04.002
91. Davies, PDO. Molecular Epidemiology Unmasks the Tubercle Bacillus: New Techniques Reveal New Aspects of Virulence. Thorax (2004) 59(4):273–4. doi:10.1136/thx.2003.020081
92. Lannerö, E, Wickman, M, Pershagen, G, and Nordvall, SL. Maternal Smoking During Pregnancy Increases the Risk of Recurrent Wheezing During the First Years of Life (BAMSE). Respir Res (2006) 7:3–6. doi:10.1186/1465-9921-7-3
93. Yang, Q, Wen, SW, Smith, GN, Chen, Y, Krewski, D, Chen, XK, et al. Maternal Cigarette Smoking and the Risk of Pregnancy-Induced Hypertension and Eclampsia. Int J Epidemiol (2006) 35(2):288–93. doi:10.1093/ije/dyi247
94. Xiong, X, Demianczuk, NN, Saunders, LD, Wang, FL, and Fraser, WD. Impact of Preeclampsia and Gestational Hypertension on Birth Weight by Gestational Age. Am J Epidemiol (2002) 155(3):203–9. doi:10.1093/aje/155.3.203
95. Pipkin, FB, Genetics of Preeclampsia Consortium. Smoking in Moderate/Severe Preeclampsia Worsens Pregnancy Outcome, But Smoking Cessation Limits the Damage. Hypertension (2008) 51:1042–6. doi:10.1161/HYPERTENSIONAHA.107.106559
96. Cnattingius, S, Mills, JL, Yuen, J, Eriksson, O, and Salonen, H. The Paradoxical Effect of Smoking in Preeclamptic Pregnancies: Smoking Reduces the Incidence But Increases the Rates of Perinatal Mortality, Abruptio Placentae, and Intrauterine Growth Restriction. Am J Obstet Gynecol (1997) 177:156–61. doi:10.1016/s0002-9378(97)70455-1
97. Miller, EC, Cao, H, Wen, SW, Yang, Q, Lafleche, J, and Walker, MC. The Risk of Adverse Pregnancy Outcomes Is Increased in Preeclamptic Women Who Smoke Compared With Non-Preeclamptic Women Who Do Not Smoke. Am J Obstet Gynecol (2010) 203(4):e1–8. Epub 2010 Jun 26. PMID: 20579958. doi:10.1016/j.ajog.2010.05.020
98. Taioli, EWEL, and Wynder, EL. Re: Endocrine Factors and Adenocarcinoma of the Lung in Women. J Natl Cancer Inst (1994) 86(11):869–70. doi:10.1093/jnci/86.11.869
99. Guaderrama, MM, Corwin, EJ, Kapelewski, CH, and Klein, LC. Sex Differences in Effects of Cigarette Smoking and 24-Hr Abstinence on Plasma Arginine Vasopressin. Addict Behav (2011) 36(11):1106–9. doi:10.1016/j.addbeh.2011.06.015
100. Fréour, T, Dessolle, L, Lammers, J, Lattes, S, and Barrière, P. Comparison of Embryo Morphokinetics After In Vitro Fertilization-Intracytoplasmic Sperm Injection in Smoking and Nonsmoking Women. Fertil Steril (2013) 99(7):1944–50. doi:10.1016/j.fertnstert.2013.01.136
101. Freour, T, Masson, D, Dessolle, L, Allaoua, D, Dejoie, T, Mirallie, S, et al. Ovarian Reserve and In Vitro Fertilization Cycles Outcome According to Women Smoking Status and Stimulation Regimen. Arch Gynecol Obstet (2012) 285(4):1177–82. doi:10.1007/s00404-011-2172-7
102. Bennett, WP, Alavanja Brunhilde Blomeke, MCR, Va¨ha¨kangas, KH, Castre´n, K, Welsh, JA, Bowman, ED, et al. Environmental Tobacco Smoke, Genetic Susceptibility, and Risk of Lung Cancer in Never-Smoking Women. J Natl Cancer Inst (1999) 91(23):2009–14. doi:10.1093/jnci/91.23.2009
103. erkins, KA, and Scott, J. Sex Differences in Long-Term Smoking Cessation Rates Due to Nicotine Patch. Nicotine Tob Res (2008) 10(7):1245–50. doi:10.1080/14622200802097506
104. Penn, G, and Owen, L. Factors Associated With Continued Smoking During Pregnancy: Analysis of Socio-Demographic, Pregnancy and Smoking-Related Factors. Drug Alcohol Rev (2002) 21(1):17–25. doi:10.1080/09595230220119291
105. Schneider, S, and Schutz, J. Who Smokes During Pregnancy? A Systematic Literature Review of Population-Based Surveys Conducted in Developed Countries Between 1997 and 2006. Eur J Contracept Reprod Heal Care (2008) 13(2):138–47. doi:10.1080/13625180802027993
106. Schneider, S, Huy, C, Schütz, J, and Diehl, K. Smoking Cessation During Pregnancy: A Systematic Literature Review. Drug Alcohol Rev (2010) 29(1):81–90. doi:10.1111/j.1465-3362.2009.00098.x
107. Connor, SK, and McIntyre, L. The Sociodemographic Predictors of Smoking Cessation Among Pregnant Women in Canada. Can J Public Heal (1999) 90(5):352–5. doi:10.1007/BF03404527
108. Colman, GJ, and Joyce, T. Trends in Smoking Before, During, and After Pregnancy in Ten States. Am J Prev Med (2003) 24:29–35. doi:10.1016/S0749-3797(02)00574-3
Keywords: cigarette smoking, tobacco dependence, health effect, sex, female
Citation: Yang L, Zhou Y, Jiang M, Wen W, Guo Y, Pakhale S and Wen SW (2024) Why Female Smokers Have Poorer Long-Term Health Outcomes than Male Smokers: The Role of Cigarette Smoking During Pregnancy. Public Health Rev 45:1605579. doi: 10.3389/phrs.2024.1605579
Received: 11 November 2022; Accepted: 11 January 2024;
Published: 29 February 2024.
Edited by:
Ana Ribeiro, University Porto, PortugalReviewed by:
Saeed Anwar, University of Alberta, CanadaAna Cruz, University of Porto, Portugal
One reviewer who chose to remain anonymous
Copyright © 2024 Yang, Zhou, Jiang, Wen, Guo, Pakhale and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
PHR is edited by the Swiss School of Public Health (SSPH+) in a partnership with the Association of Schools of Public Health of the European Region (ASPHER)+
*Correspondence: Smita Pakhale, c3Bha2hhbGVAdG9oLmNh; Shi Wu Wen, c3d3ZW5Ab2hyaS5jYQ==
†These authors have contributed equally to this work
 Li Yang1,2†
Li Yang1,2† Yunchun Zhou
Yunchun Zhou Shi Wu Wen
Shi Wu Wen