Abstract
Objective:
This study aimed to assess incidence and predictors of mortality among preterm neonates in Jimma University Medical Center, Southwest Ethiopia.
Methods:
A retrospective follow-up study was conducted among 505 preterm neonates admitted to the Neonatal Intensive Care Unit of Jimma University Medical Center from 01 January 2017, to 30 December 2019. Data were collected from medical records using a data collection checklist. Data were entered into Epi-Data 3.1 and analyzed with STATA 15. Cox-regression analysis was fitted to identify predictors of preterm neonatal mortality. Variables with p-value <0.05 were declared a statistical significance.
Result:
The cumulative incidence of preterm neonatal death was 25.1%. The neonatal mortality rate was 28.9 deaths (95%CI: 24.33, 34.46) per 1,000 neonate-days. Obstetric complications, respiratory distress syndrome, neonatal sepsis, perinatal asphyxia, antenatal steroid exposure, gestational age at birth, and receiving kangaroo-mother care were predictors of preterm neonatal mortality.
Conclusion:
Preterm neonatal mortality rate was high. Hence, early detection and management of obstetric and neonatal complications, use of antenatal steroids, and kangaroo-mother care should be strengthened to increase preterm neonatal survival.
Introduction
Preterm birth, births earlier than 37 weeks of gestational age, is a global public health priority that is linked with high neonatal morbidity and mortality, mainly in developing countries [1–4]. The preterm birth rate is increasing and great inequalities exist in a quality, access to care, and survival across countries [1]. The risk of dying is highest in the first 4 weeks of life for all babies, but preterm babies are acutely so and they need special care just to remain alive [1, 3].
Preterm birth affects nearly fifteen million people worldwide each year, with a rate of around 11% [1]. Prematurity is the leading cause of neonatal mortality and the second leading cause of death among children under the age of five worldwide. Prematurity is also the leading cause of multiple health risks in both the short and long term [1, 3]. Asia and Sub-Saharan Africa accounted for nearly 80% of all preterm births [5].
More than 35% of all neonatal mortality globally results from preventable and treatable preterm birth complications [3, 4, 6]. Nearly one million neonates die each year from preterm birth complications [7]. The survival chance of preterm neonates varies significantly based on where they were born. More than three-fourths (75%) of preterm babies could be saved with the feasible and cost-effective practice of quality care, and further reductions are possible with intensive neonatal care [1, 4].
The consequence of being born preterm extends beyond the neonatal period. They need proper care and treatment as they face greater risks of lifetime disability as well as a deprived quality of life [1, 7]. Moreover, mothers of preterm neonates experience significant psychological distress and families also endure substantial financial hardship [8, 9]. Prematurity is associated with higher healthcare costs, particularly within the first year after birth, suggesting that the implementation of appropriate programs and strategies to prevent premature delivery is beneficial from a medical as well as a healthcare expenses perspective [10].
Different findings identified mainly that mother and her neonate socio-demographic factors, maternal medical-related factors, and obstetric and gynecologic-related factors as the predictors of mortality among preterm neonates [11–18]. Ethiopia was one of the top ten countries with a high burden of preterm births globally. In Ethiopia, more than three hundred thousand neonates were born prematurely every year, and the rate of preterm birth was 12% [5, 19]. As a result, Ethiopia has adopted the new WHO recommendations for improving preterm neonatal outcomes in clinical practice [20]. Besides, the country has developed a national newborn and child survival strategy from 2015 to 2020 which aims to improve the survival of neonates, mainly preterm neonates, through the inclusion of high-impact life-saving neonatal interventions and intends to end all preventable newborn and child deaths by 2035 [21]. Despite these efforts, prematurity is the first leading cause of neonatal mortality and the fourth leading cause of mortality among children below the age of five in Ethiopia [22, 23]. Conversely, sustainable development goals (SGDs) three place a high priority on reducing newborn mortality, with a target of 12 neonatal deaths per 1,000 live births by 2030 [24]. Hence, prematurity should be addressed to curb neonatal death globally and attain SDGs [5].
There is a dearth of recent evidence on incidence and predictors of mortality among preterm neonates to inform programs and policies in Ethiopia, particularly in a study area. This significantly limits understanding of the extent and depth of the problem for evidence-based intervention. It is a dual agenda to prevent preterm delivery and address the survival gap of preterm neonates which necessitates comprehensive research to end the preventable deaths of neonates and children below 5 years. The study will help healthcare providers to identify the main predictors of preterm neonatal mortality and intervention areas, and in the timely detection of high-risk babies to give maximum efforts for their survival. Hence, this study aimed to assess the incidence and predictors of mortality among preterm neonates admitted to neonatal intensive care unit (NICU) in Jimma University Medical Center (JUMC) in Southwest Ethiopia.
Methods
Study Design, Period, and Setting
A retrospective follow-up study was conducted among preterm neonates admitted to NICU at JUMC between 1 January 2017, and 30 December 2019. JUMC is found in Jimma town 352 km away from Addis Ababa, the capital city of Ethiopia, in the southwestern part of the country. JUMC currently provides a range of services for approximately 15 million people. The NICU unit is one of the intensive care unit services currently in operation at the hospital which has 26 neonatal and 16 kangaroo-mother care beds. The unit also has 20 radiant warmers, four continuous positive airway pressure (CPAP), six photo-therapy machines, oxygen concentrator machines, pulse oximetry, a glucometer, and neonatal resuscitation equipment. On average, nearly 1,500 neonates are admitted annually to NICU of JUMC. The functional capability of JUMC is level three NICU [25]. The data collection period was from March 11 to 20 April 2020.
Population
The source population for this study was all preterm neonates admitted to the NICU of JUMC from 1 January 2017, to 30 December 2019. All randomly selected preterm neonates admitted to the NICU of JUMC from 1 January 2017, to 30 December 2019, and fulfilling the eligibility criteria were the study population. All alive-born preterm neonates at admission who were registered on the neonatal registry book from 1 January 2017, to 30 December 2019, in the NICU of JUMC were included in the study. However, preterm neonates with incomplete information on medical records regarding outcome status, a time when neonates were admitted to NICU, and a time when death or censoring occurred were excluded.
Sample Size Determination and Sampling Procedure
The sample size was determined for survival analysis by considering preterm neonates who have jaundice using STATA Version 15 statistical software based on the following assumptions: 5% level of significance (α) (two-sided), 80% power, adjusted hazard ratio of 1.62 for preterm neonates who have jaundice, overall probability of preterm neonatal death (d) of 0.288 [15], and 0.5 variabilities of covariates of interest. It was assumed that no subjects were anticipated to withdraw from the follow-up, and a 10% contingency was added for incomplete records. Hence, the total sample size for this study was 516.
The medical registration number (MRN) of preterm neonates over three 3-year period from 1 January 2017, to 30 December 2019, was taken from the NICU logbook to create a sampling frame. A total of 957 preterm neonates were admitted to NICU. A computer-generated simple random sampling technique was employed to select 516 participants for the study as follows: The sampling frame that was created using the MRN was entered into SPSS version 25 software. Then, a 516 sample was selected randomly using the SPSS select case procedure. Medical records of preterm neonates attached to selected MRNs were reviewed, and those records that met eligibility criteria were included in the analysis.
Study Variables
Time to death of preterm neonates was the dependent variable (death was an event and coded “1,” and censored observation coded “0”). Socio-demographic related variables such as sex of neonate, neonatal age at admission, maternal age, and residence; maternal medical condition-related variables like maternal febrile illness/disease, anemia, diabetes mellitus, and human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS); obstetric and gynecologic related variables like gravidity, antenatal care visit, birth type, history of bad obstetric and/or gynecologic outcome, mode of delivery, presentation, place of delivery, antenatal steroid use, and obstetric complications; and preterm neonates related variables such as gestational age at birth, birth weight, weight class for gestational age, fifth minute APGAR score, kangaroo-mother care, initiation of breastfeeding, method of feeding, congenital malformation, temperature, respiratory distress syndrome, neonatal sepsis, perinatal asphyxia, hypoglycemia, anemia, and jaundice were independent variables.
Data Collection Instrument, Personnel, and Procedure
Data were collected from preterm neonatal medical records and registers by three trained bachelor’s degree holder midwives and supervised by one bachelor’s degree holder senior nurse. A data collection checklist adapted from the Global Neonatal Database data collection form for the Ethiopian Neonatal Network was used to collect the data [26]. Modifications were made to the checklist based on NICU registration format, and through reviewing relevant literature. The starting point for follow-up was the first NICU admission date and followed until the last neonatal period (28th days of life), which was the endpoint of the study.
Operational Definitions
- ➢
Survival status: An outcome of the neonate during follow-up from the medical records and is considered as “death” if neonate died during follow-up, as “lost to follow-up” if the mother or caregiver was not available and unable to reach their address. It was considered as “Withdrawal” if the mother refused the follow-up due to inconvenience, as “refereed” if the neonate was referred to other institutions for better management, and “alive” if the preterm neonate survival was assured at the last follow-up period [27].
- ➢
Censored: Preterm neonates who were alive at the end of follow-up, lost to follow-up, withdrawal, or referred to other health institutions without knowing the outcome status [27].
- ➢
Survival time: Measure of the follow-up time (in days) from the date of admission in the NICU up to the date of death, censored, or the end of the study (28th day of life) [28].
- ➢
Time-to-death: Death of a preterm neonate on a specific day in the first 28 days of life [27].
Data Quality Assurance
Data quality was assured by the careful designing of the data collection checklist, recruiting data collectors, and supervisor who have previous experience. A preliminary chart review was done on 26 randomly selected records (5% of the sample size) before the commencement of the actual study, and relevant clarifications and amendments were taken on the checklist. Training for 2 days was given on principles of research ethics, data collection checklist, and procedures for data collectors and a supervisor. Data collectors were supervised closely by the supervisor daily throughout the data collection period.
Data Processing and Analysis
Data were cleaned, coded, and entered into Epi-Data version 3.1, and analysis was done using STATA version 15.0. Descriptive statistics such as frequencies, percentages, summary measures, and rates were computed to describe categorical and continuous variables as supposed necessary. The incidence rate of neonatal mortality was computed by dividing the number of preterm neonates who died during the follow-up period by the total neonate-days at risk of observation. The Kaplan-Meier (KM) method was used to estimate median survival time and compare survival experience between categories of variables. Log-rank test was used to compare statistically significant differences in survival experience among groups.
The Cox proportional hazard model was used to identify predictors of time to death. A bivariable cox-proportional hazard model was fitted first, and variables with a p-value <0.25 in this analysis entered into the multivariable cox-proportional hazard model. To identify independent predictors of time to death, a stepwise backward likelihood ratio method was used to fit a multivariable cox-proportional hazard model. An adjusted hazard ratio with a 95% confidence interval was computed to determine the strength of the association. Variables with a p-value <0.05 in the final model were considered significant predictors of the time to death of preterm neonates.
The proportional hazard assumption was checked by the Schoenfeld residual test and was satisfied (Global test X2 = 5.13, p-value = 0.92). Multicollinearity was checked by looking at the variance inflation factor (VIF) and the highest observed VIF value was 2.06, indicating that there was no multicollinearity threat. The goodness of model fitness was evaluated by using the Cox-Snell residual test. In this study, the Nelson-Aalen cumulative hazard function follows the 45° diagonal line very closely, indicating that it almost has an exponential distribution with a hazard rate of one. Hence, for the residual test, it was possible to conclude that the final model fits the data very well (Figure 1).
FIGURE 1
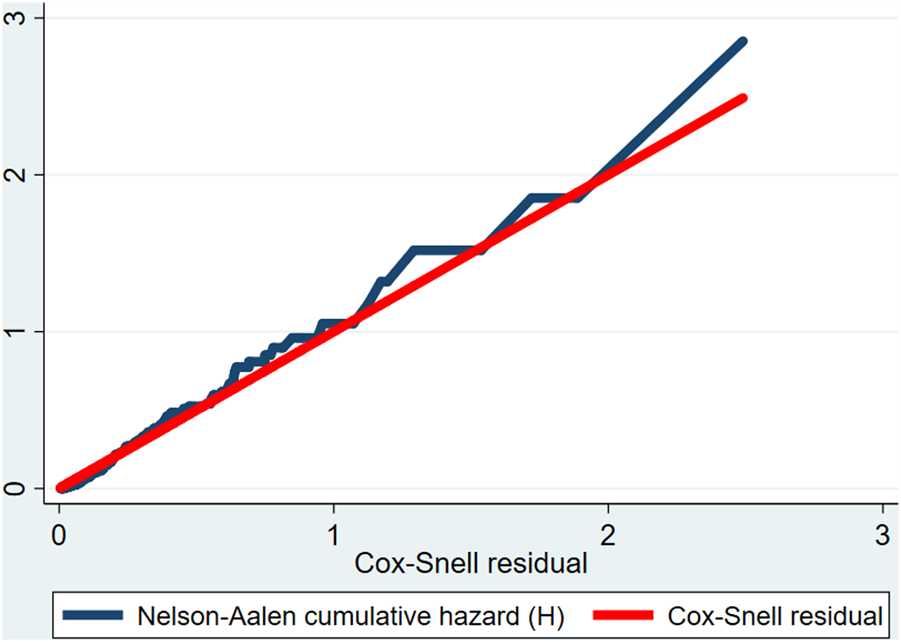
Cox-Snell residual Nelson-Aalen cumulative hazard function among preterm neonates admitted to the Neonatal Intensive Care Unit of Jimma University Medical Center, Southwest Ethiopia, 2020.
Results
A total of 516 preterm neonate medical records were reviewed, and 505 records that met eligibility criteria were included in the analysis with a response rate of 97.8%. Eleven medical records which did not fulfil the eligibility criteria were excluded. From excluded records, 6 records had incomplete data on outcome status, 3 records had an unknown date when the outcome of interest happened, and 2 records had an unknown date of admission to NICU (Supplementary Material S1).
Socio-Demographic Characteristics
Near to nine-tenth of neonates, 433 (85.7%), had less than 24 h of age at admission and more than half, 279 (55.2%), of them were males. The median age of the mother was 27 years (an interquartile range (IQR) of 8 years). Most mothers of the neonates, 398 (78.8%), were between the ages of 20 and 34. Nearly two-thirds, 339 (67.1%), of preterm neonates were rural residents (Table 1).
TABLE 1
| Variable | Categories | Survival status | Total (%) | |
|---|---|---|---|---|
| Death N (%) | Censored N (%) | |||
| Neonatal age at admission | <1 day 1–6 days ≥7 days |
109 (25.2) 17 (26.6) 1 (12.5) |
324 (74.8) 47 (73.4) 7 (87.5) |
433 (85.7) 64 (12.7) 8 (1.6) |
| Sex of neonate | Male Female |
74 (26.5) 53 (23.5) |
205 (73.5) 173 (76.5) |
279 (55.2) 226 (44.8) |
| Age of the mother (in years) | <20 20–34 ≥35 |
3 (30.0) 93 (23.4) 31 (32.0) |
7 (70.0) 305 (76.6) 66 (68.0) |
10 (2.0) 398 (78.8) 97 (19.2) |
| Residence | Rural Urban |
87 (25.7) 40 (24.1) |
252 (74.3) 126 (75.9) |
339 (67.1) 166 (32.9) |
Socio-demographic characteristics of preterm neonates and their mothers at Neonatal Intensive Care Unit of Jimma University Medical Center, Jimma, Southwest Ethiopia, 2020 (N = 505).
Maternal Medical, Obstetric, and Gynecologic Characteristics
Of the participants, 66 (13.1%) mothers had known or been diagnosed with a medical disease, and more than nine-tenths of the mothers, 467 (92.5%), had antenatal care visits during the current pregnancy. Almost a quarter, 124 (24.6%), of the mothers had used antenatal steroids. Nearly three-fourths, 372 (73.7%), of the mothers had spontaneous onset of labor, and the majority of the delivery, 462 (91.5%), had a cephalic presentation. Almost half, 250 (49.5%), of the mothers had an obstetric complication (Table 2).
TABLE 2
| Variable | Categories | Survival status | Total (%) | |
|---|---|---|---|---|
| Death N (%) | Censored N (%) | |||
| Known or diagnosed medical diseases | Yes No |
20 (30.3) 107 (24.4) |
46 (69.7) 332 (75.6) |
66 (13.1) 439 (86.9) |
| Febrile illness or disease | Yes No |
11 (33.3) 116 (24.6) |
22 (66.7) 356 (75.4) |
33 (6.5) 472 (93.5) |
| Anemia | Yes No |
7 (33.3) 120 (24.8) |
14 (66.7) 364 (75.2) |
21 (4.2) 484 (95.8) |
| Othersa | Yes No |
3 (15.8) 124 (25.5) |
16 (84.2) 362 (74.5) |
19 (3.8) 486 (96.2) |
| Gravidity | I II-IV ≥V |
38 (22.4) 64 (25.4) 25 (30.1) |
132 (77.6) 188 (74.6) 58 (69.9) |
170 (33.7) 252 (49.9) 83 (16.4) |
| Antenatal care visit | Yes No |
108 (23.1) 19 (50.0) |
359 (76.9) 19 (50.0) |
467 (925) 38 (7.5) |
| Birth type | Single Twin |
91 (25.3) 36 (24.8) |
269 (74.7) 109 (75.2) |
360 (71.3) 145 (28.7) |
| Bad obstetric and/or gynecologic historyb | Yes No |
38 (32.2) 89 (23.0) |
80 (67.8) 298 (77.0) |
118 (23.4) 387 (76.6) |
| Antenatal steroid use | Yes No |
22 (17.7) 105 (27.6) |
102 (82.3) 276 (72.4) |
124 (24.6) 381 (75.4) |
| Mode of delivery | Spontaneous vaginal delivery Assisted vaginal delivery Cesarean section (C/S) |
79 (23.5) 3 (14.3) 45 (30.4) |
257 (76.5) 18 (85.7) 103 (69.6) |
336 (66.5) 21 (4.2) 148 (29.3) |
| Cause of onset of labor | Spontaneous Induced C/S |
89 (23.9) 12 (29.3) 26 (28.3) |
283 (76.1) 29 (70.7) 66 (71.7) |
372 (73.7) 41 (8.1) 92 (18.2) |
| Presentation | Cephalic Non-cephalic |
116 (25.1) 11 (25.6) |
346 (74.9) 32 (74.4) |
462 (91.5) 43 (8.5) |
| Place of delivery | Hospital Health center Home |
97 (24.9) 20 (23.3) 9 (34.6) |
292 (75.1) 69 (76.7) 17 (65.4) |
389 (77.0) 90 (17.8) 26 (5.2) |
| Obstetric complications | Yes No |
75 (30.0) 52 (20.4) |
175 (70.0) 203 (79.6) |
250 (49.5) 255 (50.5) |
| Preeclampsia | Yes No |
23 (28.8) 104 (24.5) |
57 (71.2) 321 (75.5) |
80 (15.8) 425 (84.2) |
| Eclampsia | Yes No |
8 (32.0) 119 (24.8) |
17 (68.0) 361 (75.2) |
25 (5.0) 480 (95.0) |
| Fetal distress | Yes No |
26 (32.1) 101 (23.8) |
55 (67.9) 323 (76.2) |
81 (16.0) 424 (84.0) |
| Preterm premature rupture of membrane | Yes No |
20 (32.3) 107 (24.2) |
42 (67.7) 336 (75.8) |
62 (12.3) 443 (87.7) |
| Abruption placenta | Yes No |
6 (18.7) 121 (25.6) |
26 (81.3) 352 (74.4) |
32 (6.3) 473 (93.7) |
| Placenta Previa | Yes No |
8 (34.8) 119 (24.7) |
15 (65.2) 363 (75.3) |
23 (4.6) 482 (95.4) |
| Chorioamnionitis | Yes No |
9 (32.1) 118 (24.7) |
19 (67.9) 359 (75.3) |
28 (5.5) 477 (94.5) |
| Othersc | Yes No |
10 (41.7) 117 (24.3) |
14 (58.3) 364 (75.7) |
24 (4.8) 481 (95.2) |
Maternal medical, obstetric, and/or gynecologic characteristics of a study participant in the Neonatal Intensive Care Unit of Jimma University Medical Center, Jimma, Southwest Ethiopia, 2020 (N = 505).
Diabetes mellitus, HIV/AIDS, cardiac disease, renal disease, and STIs.
Neonatal death, stillbirth, abortion, and intrauterine fetal death.
Cord prolapse, oligohydramnios, polyhydramnios, and prolonged labor.
Preterm Neonate-Related Characteristics
Almost four-fifths of the neonates, 399 (79%), were moderate preterm, and more than two-thirds of neonates, 349 (69%), had low birth weight. Out of the cohort, 454 (89.9%) had not initiated breastfeeding within 1 hour of birth. More than three-fourths of neonates, 397 (78.6%), were diagnosed with hypothermia followed by respiratory distress syndrome 295 (58.4%), and hypoglycemia 167 (33.1%). Kangaroo-mother care (KMC) was provided to nearly one in every ten neonates (30.7%). More than three-fifths of neonates, 59.6%, received nasal CPAP (Table 3).
TABLE 3
| Variable | Categories | Survival status | Total (%) | |
|---|---|---|---|---|
| Death N (%) | Censored N (%) | |||
| Gestational age at birth (in weeks) | <28 28- <32 32- <37 |
14 (70.0) 34 (39.5) 79 (19.8) |
6 (30.0) 52 (60.5) 320 (80.2) |
20 (4.0) 86 (17.0) 399 (79.0) |
| Birth weight (in grams) | <1,000 1,000–1,499 1,500–2,499 ≥2,500 |
15 (62.5) 40 (36.7) 69 (19.8) 3 (13.0) |
9 (37.5) 69 (63.3) 280 (80.2) 20 (87.0) |
24 (4.8) 109 (21.6) 349 (69.0) 23 (4.6) |
| Weight class for gestational age | AGA SGA LGA |
122 (25.3) 5 (29.4) 0 (0.0) |
360 (74.7) 12 (70.6) 6 (100.0) |
482 (95.4) 17 (3.4) 6 (1.2) |
| Fifth-minute APGAR score | <7 ≥7 |
41 (38.7) 86 (21.6) |
65 (61.3) 313 (78.4) |
106 (21.0) 399 (79.0) |
| Initiation of breastfeeding within 1 hour of birth | Yes No |
5 (9.8) 122 (26.9) |
46 (90.2) 332 (73.1) |
51 (10.1) 454 (89.9) |
| Method of feeding | Breast sucking NGT Cup feeding |
26 (18.6) 99 (28.2) 2 (14.3) |
114 (81.4) 252 (71.8) 12 (85.7) |
140 (27.7) 351 (69.5) 14 (2.8) |
| Hypothermia | Yes No |
102 (25.7) 25 (23.1) |
295 (74.3) 83 (76.9) |
397 (78.6) 108 (21.4) |
| Respiratory distress syndrome | Yes No |
93 (31.5) 34 (16.2) |
202 (68.5) 176 (83.8) |
295 (58.4) 210 (41.6) |
| Neonatal sepsis | Yes No |
51 (33.8) 76 (21.5) |
100 (66.2) 278 (78.5) |
151 (29.9) 354 (70.1) |
| Perinatal asphyxia | Yes No |
17 (42.5) 110 (23.7) |
23 (57.5) 355 (76.3) |
40 (7.9) 465 (92.1) |
| Hypoglycemia | Yes No |
47 (28.1) 80 (23.7) |
120 (71.9) 258 (76.3) |
167 (33.1) 338 (66.9) |
| Anemia | Yes No |
10 (29.4) 117 (24.8) |
24 (70.6) 354 (75.2) |
34 (6.7) 471 (93.2) |
| Jaundice | Yes No |
45 (36.9) 82 (21.4) |
77 (63.1) 301 (78.6) |
122 (24.2) 383 (75.8) |
| Congenital malformation | Yes No |
8 (36.4) 119 (24.6) |
14 (63.6) 364 (75.4) |
22 (4.4) 483 (95.6) |
| Apnea of prematurity | Yes No |
8 (50.0) 119 (24.3) |
8 (50.0) 370 (75.7) |
16 (3.2) 489 (96.8) |
| Others* | Yes No |
3 (10.3) 124 (26.1) |
26 (89.7) 352 (73.9) |
29 (5.7) 471 (94.3) |
| Received kangaroo mother care | Yes No |
22 (14.2) 105 (30.0) |
133 (85.8) 245 (70.0) |
155 (30.7) 350 (69.3) |
| Received nasal CPAP | Yes No |
85 (28.2) 42 (20.6) |
216 (71.8) 162 (79.4) |
301 (59.6) 204 (40.4) |
| Resuscitated with a bag and mask | Yes No |
43 (27.0) 84 (24.3) |
116 (73.0) 262 (75.7) |
159 (31.5) 346 (68.5) |
| Received phototherapy | Yes No |
36 (31.9) 91 (23.2) |
77 (68.1) 301 (76.8) |
113 (22.4) 392 (77.6) |
| Heated with radiant warmer | Yes No |
98 (25.6) 29 (23.8) |
285 (74.4) 93 (76.2) |
383 (75.8) 122 (24.2) |
Neonatal-related characteristics of preterm neonate admitted to Neonatal Intensive Care Unit of Jimma University Medical Center, Jimma, Southwest Ethiopia, 2020 (N = 505).
Meningitis, ophthalmic neonatorum, necrotizing enterocolitis, pulmonary hypertension, HIV, exposed, meconium aspiration syndrome, hospital-acquired infection, and birth trauma.
Incidence of Mortality Among Preterm Neonate
During the follow-up, the cumulative incidence of preterm neonatal death was 127 (25.1%). Of all deaths, 15.7% died in the first 24 h of life, and 81.1% of deaths occurred within 7 days of life. Out of the cohort, 352 (69.7%) improved and were discharged to home, 15 (3.0%) lost to follow-up, 6 (1.2%) were referred to other hospitals, and the remaining 5 (1.0%) were withdrawn from the follow-up.
A cohort contributed a total of 4,386 neonate days at risk of observation. The overall neonatal mortality rate (incidence density) was 28.9 deaths per 1,000 neonate-days (95% CI: 24.33, 34.46). The neonatal mortality rate (NMR) was 67.3 deaths per 1,000 neonate-days in the first 24 h of life (95% CI: 48.11, 94.23). Early NMR (death within 7 days of life) was 40 deaths per 1,000 neonate-days (95% CI: 33.08, 48.33); however, the late NMR was 11.7 deaths per 1,000 neonate-days (95% CI: 7.55, 18.13).
Overall Survival Function
Preterm neonates were followed for different periods: a minimum of 1 day and a maximum of 28 days. The overall median length of follow-up was 7 (IQR = 8) days. The cumulative survival probability at the end of the follow-up was 54.94% (95% CI: 41.83, 66.27). The cumulative probability of survival at the end of the first, seventh, 14th, and 21st days was 93.27% (95% CI: 90.71, 95.14), 76.89% (95% CI: 72.73, 80.51), 71.8% (95% CI: 66.79, 76.19), and 66.96% (60.54, 72.58), respectively. The overall mean survival time was 20.42 neonate days (95% CI: 19.27, 21.56).
Survival Function and Comparison of Survival Experience
Preterm neonates born from mothers who used antenatal steroids had higher survival experiences compared to their counterparts (chi-square = 5.17, p-value = 0.023). Likewise, preterm neonates who received KMC had a higher survival experience than neonates who didn’t receive KMC (chi-square = 14.18, p-value = 0.0002). However, preterm neonates born from mothers with obstetric complications had lower survival experience than their counterparts (chi-square = 11.71, p-value = 0.001). Neonates having respiratory distress syndrome (RDS) had lower survival experiences than neonates without RDS (chi-square = 11.14, p-value = 0.001). Preterm neonates with neonatal sepsis had lower survival experiences than their complements (chi-square = 7.55, p-value = 0.006). Neonates with perinatal asphyxia (PNA) had lower survival experiences than their counterparts (chi-square = 7.51, p-value = 0.003) (Figure 2).
FIGURE 2
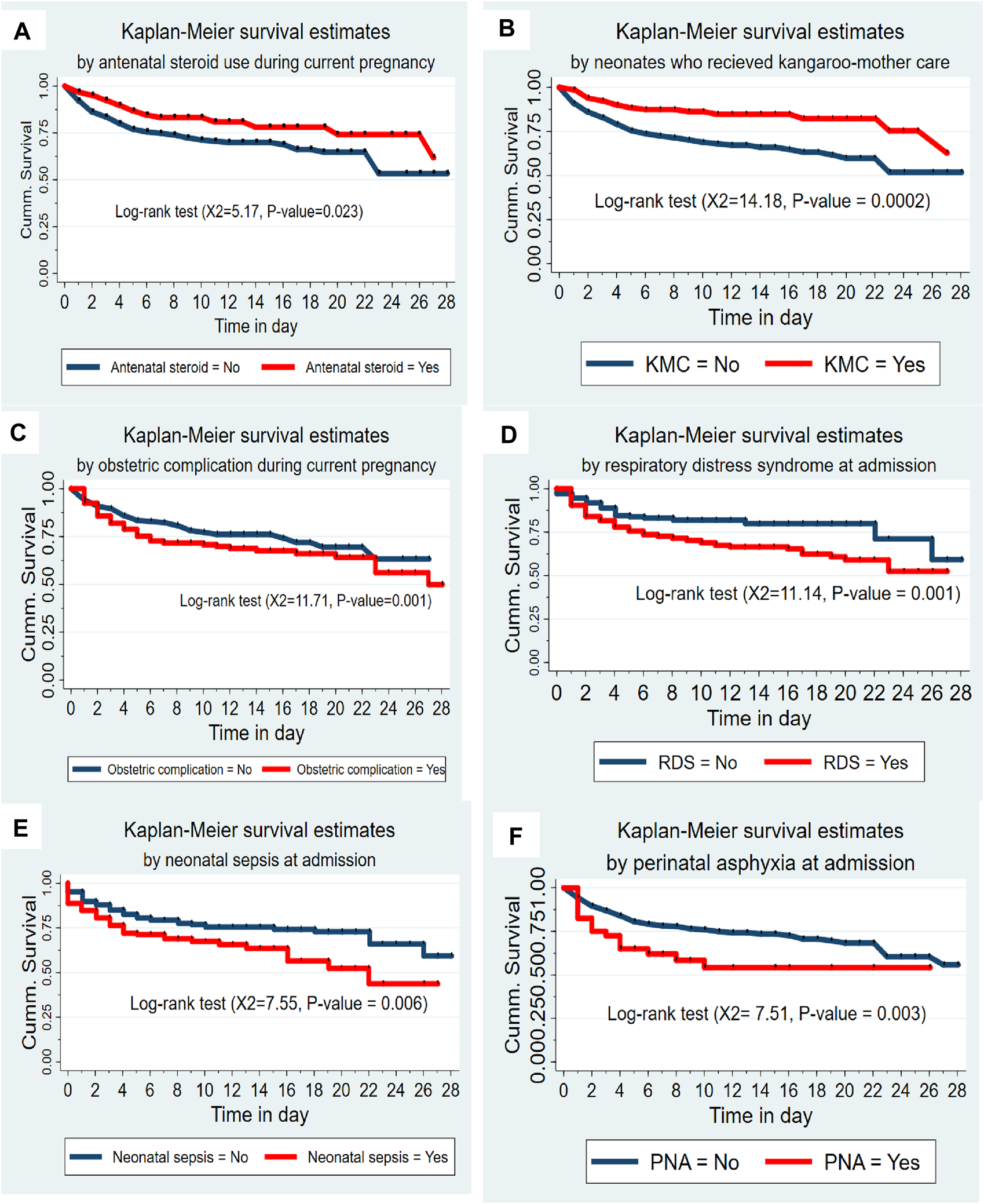
(A) The Kaplan-Meier survival curves by antenatal steroid use among preterm neonates admitted to the Neonatal Intensive Care Unit at Jimma University Medical Center, Southwest Ethiopia, 2020 (n = 505). (B) The Kaplan-Meier survival curves by KMC service among preterm neonates admitted to the Neonatal Intensive Care Unit at Jimma University Medical Center, Southwest Ethiopia, 2020 (n = 505). (C) The Kaplan-Meier survival curves by obstetric complication among preterm neonates admitted to the Neonatal Intensive Care Unit at Jimma University Medical Center, Southwest Ethiopia, 2020 (n = 505). (D) The Kaplan-Meier survival curves by respiratory distress syndrome among preterm neonates admitted to the Neonatal Intensive Care Unit at Jimma University Medical Center, Southwest Ethiopia, 2020 (n = 505). (E) The Kaplan-Meier survival curves by neonatal sepsis among preterm neonates admitted to the Neonatal Intensive Care Unit at Jimma University Medical Center, Southwest Ethiopia, 2020 (n = 505). (F) The Kaplan-Meier survival curves by perinatal asphyxia among preterm neonates admitted to the Neonatal Intensive Care Unit at Jimma University Medical Center, Southwest Ethiopia, 2020 (n = 505).
Predictors of Preterm Neonatal Mortality
In the multivariable cox-regression model; antenatal steroid use, obstetric complication during the current pregnancy, an increment in gestational age at birth, receiving KMC, having RDS, neonatal sepsis, and PNA were found to be predictors for time to death of preterm neonates at p-value <0.05.
Preterm neonates born from mothers who used antenatal steroids during current pregnancy had 45% fewer hazard of death compared to neonates born from mothers who didn’t use antenatal steroids (AHR = 0.55; 95% CI:0.34, 0.90). Preterm neonates born from mothers with an obstetric complication had a 1.84 times higher hazard of death compared to those who were born from mothers without obstetric complication (AHR = 1.84; 95% CI: 1.20, 2.82).
As the gestational age of preterm neonates at birth increases by 1 week, the hazard of death decreases by 19% (AHR = 0.81; 95% CI: 0.75, 0.87). Preterm neonates who had RDS had 1.52 times more hazard of death than those without RDS (AHR = 1.52; 95% CI:1.01, 2.29). Preterm neonates who had neonatal sepsis had about 1.71 times greater hazard of death than neonates without neonatal sepsis (AHR = 1.71; 95% CI: 1.18, 2.49). Preterm neonates who had PNA had 2.44 times more hazard of death compared to those neonates without PNA (AHR = 2.44; 95% CI: 1.33, 4.49). Preterm neonates who received KMC had 52% lesser hazard of death as compared to their counterparts (AHR = 0.48; 95% CI: 0.30, 0.77) (Table 4).
TABLE 4
| Variables | Survival status | CHR [95% CI] | p-value | AHR [95% CI] | p-value | |
|---|---|---|---|---|---|---|
| Death n (%) | Censored n (%) | |||||
| Age of the mother | ||||||
| <20 years 20–34 years ≥35 years |
3 (30.0) 93 (23.4) 31 (32.0) |
7 (70.0) 305 (76.6) 66 (68.0) |
1.50 [0.47–4.73] 1 1.43 [0.95–2.15] |
0.49 0.087 |
3.04 [0.88–10.54] 1 1.11 [0.73–1.69] |
0.079 0.62 |
| Antenatal care visit during the current pregnancy | ||||||
| Yes No |
108 (23.1) 19 (50.0) |
359 (76.9) 19 (50.0) |
1 2.25 [1.38–3.67] |
0.001 | 1 1.29 [0.75–2.23] |
0.35 |
| History of bad obstetric and/or gynecologic outcomes | ||||||
| Yes No |
38 (32.2) 89 (23.0) |
80 (67.8) 298 (77.0) |
1.33 [0.91–1.95] 1 |
0.14 | 0.91 [0.60–1.34] 1 |
0.64 |
| Antenatal steroid use during the current pregnancy | ||||||
| Yes No |
22 (17.7) 105 (27.6) |
102 (82.3) 276 (72.4) |
0.59 [0.38–0.94] 1 |
0.028 | 0.55 [0.34–0.90] 1 |
0.018 |
| Place of delivery | ||||||
| Hospital Health center Home |
97 (24.9) 20 (29.0) 9 (34.6) |
292 (75.1) 69 (71.0) 17 (65.4) |
1 1.04 [0.65–1.67] 1.68 [0.84–3.33] |
0.87 0.14 |
1 1.64 [0.95–2.82] 2.07 [0.99–4.32] |
0.074 0.051 |
| Obstetric complications during the current pregnancy | ||||||
| Yes No |
75 (30.0) 52 (20.4) |
175 (70.0) 203 (79.6) |
1.44 [1.01–2.05] 1 |
0.045 | 1.84 [1.20–2.82] 1 |
0.005 |
| Gestational age at birth (in a week) | 0.83 [0.77–0.89] | <0.001 | 0.81 [0.75–0.87] | <0.001 | ||
| Birth weight (in grams) | ||||||
| <1,000 1,000- <1,500 1,500- <2,500 ≥2,500 |
15 (62.5) 40 (36.7) 69 (19.8) 3 (15.0) |
9 (37.5) 69 (63.3) 280 (80.2) 20 (85.0) |
5.02 [1.40–17.40] 2.19 [0.68–7.09] 1.44 [0.45–4.57] 1 |
0.011 0.19 0.54 |
1.46 [0.31–6.88] 1.66 [0.46–5.98] 1.29 [0.39–4.24] 1 |
0.63 0.44 0.68 |
| Fifth-minute APGAR score | ||||||
| <7 ≥7 |
41 (38.7) 86 (21.6) |
65 (61.3) 313 (78.4) |
1.86 [1.28–2.70] 1 |
0.001 | 1.44 [0.95–2.18] 1 |
0.085 |
| Breastfeeding initiated within 1 hour of birth | ||||||
| Yes No |
5 (9.8) 122 (26.9) |
46 (90.2) 332 (73.1) |
1 2.50 [1.02–6.09] |
0.046 | 1 1.63 [0.64–4.18] |
0.31 |
| Neonates who had respiratory distress syndrome | ||||||
| Yes No |
93 (31.5) 34 (16.2) |
202 (68.5) 176 (83.8) |
1.90 [1.29–2.82] 1 |
0.001 | 1.52 [1.01–2.29] 1 |
0.045 |
| Neonates who had neonatal sepsis | ||||||
| Yes No |
51 (33.8) 76 (21.5) |
100 (66.2) 278 (78.5) |
1.62 [1.14–2.31] 1 |
0.008 | 1.71 [1.18–2.49] 1 |
0.005 |
| Neonates who had perinatal asphyxia | ||||||
| Yes No |
17 (42.5) 110 (23.7) |
23 (57.5) 355 (76.3) |
1.99 [1.19–3.32] 1 |
0.009 | 2.44 [1.33–4.49] 1 |
0.004 |
| Neonates who had jaundice | ||||||
| Yes No |
45 (36.9) 82 (21.4) |
77 (63.1) 301 (78.6) |
1.63 [1.13–2.34] 1 |
0.009 | 1.44 [0.99–2.10] | 0.057 |
| Neonates who received kangaroo mother care | ||||||
| Yes No |
22 (14.2) 105 (30.0) |
133 (85.8) 245 (70.0) |
0.43 [0.27–0.68] 1 |
<0.001 | 0.48 [0.30–0.77] 1 |
0.002 |
Bivariable and multivariable cox-regression model for predictors of preterm neonatal mortality in Neonatal Intensive Care Unit of Jimma University Medical Center, Jimma, Southwest Ethiopia, 2020 (N = 505).
Abbreviations: AHR, adjusted hazard ratio; APGAR, appearance, Pulse, Grimace, Activity, and Respiration; CHR, crude hazard ratio; CI, confidence interval; CPAP, continuous positive airway pressure; C/S, caesarean section; GA, gestational age; HIV/AIDS, Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome; IQR, interquartile range; JUMC, jimma university medical center; NGT, nasogastric tube; NICU, neonatal intensive care unit; PPROM, preterm premature rupture of membrane; SDGs, Sustainable Development Goals; SSA, Sub-Saharan Africa; SVD, spontaneous vaginal delivery.
Discussion
This study showed that 25.1% of preterm neonates died during the follow-up with an overall neonatal mortality rate of 28.9 deaths per 1,000 neonate days. This finding is consistent with studies reported from Nigeria 27.7% [29], Tigray Region 32.1% with an incidence of 36.6 per 1,000 person days [14], Gondar 32.9 deaths per 1,000 neonate-days [15], and Addis Ababa 25.3% and 36.4 deaths 1,000 neonates-day [12, 30]. However, this finding is higher than studies reported from Australia 7.7% [31], China 1.9% [32] and Uganda 8% [11]. This discrepancy between studies might be explained by variation in a study setting as there might be a high quality of neonatal care in Australia and China. A study from Australia was conducted in a hospital with a level four NICU while this study was conducted in a hospital with a level three NICU. Preterm neonates born in developed countries like Australia and China might receive improved care during pre-pregnancy, pregnancy, antepartum, and postnatal periods. Partly, this disparity might result from a difference in sample size, study design, and those reported studies were multicenter studies. Conversely, this study finding is lower than studies reported from India 33.5% [33], Southern Ethiopia 47.7 deaths per 1,000 neonatal days [17], Mizan Tepi 62.15 deaths per 1,000 neonate-days [13] and Jimma, Ethiopia 34.9% [28]. This discrepancy might result from variation in study design as a study reported from India was a multicenter prospective study conducted on a large sample size. The difference could result from variations in the study settings. The inconsistency with the finding from Jimma might be due to variation in the timing of the study as there was some improvement in antenatal and delivery care from a skilled provider and institutional delivery [22]. Partly, this might result from the fact that the NICU is organized in a good manner, and access to trained healthcare providers increased comparatively since special attention was given to preterm neonates by national neonatal and child survival strategy [21]. This finding implies that ongoing commitment and interventions need to be considered by focusing preterm neonatal survival intervention/management more on the intrapartum, immediate postpartum as well as early neonatal periods.
In this study, early NMR (40 per 1,000 neonate-days) was higher as compared to late NMR (11.7 per 1,000 neonate-days). This finding is consistent with a study reported from Gondar, Ethiopia [15]. This might be attributed to the reason that most of the preterm neonatal mortality in the resource-limited setting is related to practice during the intrapartum and immediate postpartum period, the need for intensive medical care, and timely referral of high-risk neonates. But, this finding is lower than the study conducted in Addis Ababa [30]. This inconsistency could be due to a study from Addis Ababa was a multicenter prospective study conducted on a small sample and it is a setting that receives high-risk neonates referred from different regions of the country. The finding of this study shows the need to focus preterm neonatal survival interventions more on the intrapartum as well as the immediate postpartum period, and early neonatal periods.
In the current study, preterm neonates born from mothers who used antenatal steroids had a lesser hazard of mortality than those neonates born from mothers who did not use antenatal steroids. This finding is in line with studies reported in the United States [34] and China [32]. This could be explained by the fact that the administration of steroids for mothers who had imminent preterm delivery enhances fetal lung maturity and decreases the risk of developing respiratory distress syndrome and intraventricular hemorrhage, and consequently might reduce the risk of neonatal death [35]. In this study, preterm neonates born from mothers who had an obstetric complication during their current pregnancy had a higher hazard of neonatal death compared to their counterparts. This finding is comparable with studies reported from Addis Ababa [12] and Southern Ethiopia [17]. This might be explained by the fact that obstetric complications affect the pregnancy status and placental blood transfusion, and can result in preterm delivery with subsequent preterm-related life-threatening complications which might increase the hazard of neonatal death.
In this study, an increment in gestational age at birth by 1 week decreases the hazard of preterm neonatal deaths by 19%. This finding is in line with studies reported from Gondar [15], and Addis Ababa, Ethiopia [30]. A possible reason for this might be as the gestational age of the neonates at birth increases, the maturity of the fetus will be enhanced, and the risk of developing life-threatening complications related to prematurity decreases which might contribute to a reduced risk of preterm neonatal death.
In the current study, preterm neonates who had RDS had a greater hazard of neonatal mortality compared to their counterparts. This finding is consistent with studies reported from different parts of Ethiopia: Bahir Dar [18] and Jimma [28]. This might be because of similarities in settings that lack postnatal surfactant administration. Partly, it could be explained by the fact that preterm neonates had immature lungs, and might consequently develop life-threatening complications like respiratory failure. Different literatures reported that RDS was the primary cause of preterm neonatal death [36].
In this study, preterm neonates who had neonatal sepsis had a higher hazard of neonatal mortality than preterm neonates without neonatal sepsis. This finding is in line with a study reported from Jimma [28]. This might result from the fact that preterm neonates were more likely to be born with or acquire an infection because they had immature immune defences supplemented with poor calorie intake, which might increase the risk of death [37].
In the current study, preterm neonates who had PNA had a greater hazard of neonatal mortality than those preterm neonates without PNA. This finding is consistent with studies reported from China [32], Gondar [15], Bahir Dar [18], and Jimma, Ethiopia [28]. This consistency might be elucidated by similarity in study design and follow-up period. This finding might be supported by the fact that PNA can lead to hypoxia with subsequent development of acidosis, leading to hypotension and hypoxic-ischemic encephalopathy, which further compromise oxygen delivery to the brain and might increase the risk of death [38].
In the present study, a preterm neonate who received KMC had a 52% lesser hazard of neonatal mortality compared to preterm neonates who did not receive KMC. This finding was in line with studies reported from Uganda [11], Gondar [15], and Bahir Dar, Ethiopia [18]. This consistency might be due to the similarity of the study setting, study design, and sample size. The finding was reaffirmed by the fact that receiving KMC protects neonates from the risk of hypothermia by decreasing body surface area to the external environment. Partly, it might also be explained by the fact that KMC promotes early initiation of breastfeeding, and may be used even when babies on formula-fed, which helps to prevent hypoglycemia. Moreover, KMC helps to reduce neonatal mortality by protecting them from sepsis [39, 40].
This study has some limitations. Since the data were accessed from a secondary source, some important predictors such as maternal educational status, maternal nutritional status, birth interval, birth order, duration of rupture of membrane, and first-minute APGAR score were not available in the medical records and their effect on preterm neonatal mortality was not investigated. The study did not address the probable care and service-related predictors of mortality among preterm neonates due to the nature of the study design. Additionally, the study covers only JUMC which limits generalizability to other settings found in the Oromia region and Ethiopia.
Conclusion
The incidence rate of preterm neonatal mortality was found high. Most preterm neonatal mortality occurs in the early phase of the neonatal period, which requires due attention to meet the national newborn and child survival and SDG-3 in Ethiopia. Obstetric complications, respiratory distress syndrome, neonatal sepsis, and perinatal asphyxia were found to be predictors of preterm neonatal mortality. Whereas, antenatal steroid exposure, an increment in gestational age at birth, and receiving kangaroo-mother care were preventive predictors for preterm neonatal mortality. Hence, special emphasis and close follow-up are highly warranted, especially during the early neonatal period. It is better to strengthen obstetrics and use of antenatal steroids for women having an imminent preterm delivery. Early detection and management of obstetric as well as preterm neonatal complications is highly demanding. Encouraging and supporting mothers to practice kangaroo-mother care, and ensuring a continuum of care are also crucial to enhance preterm neonatal survival.
Statements
Ethics statement
Ethical approval was obtained from the Institutional Review Board (IRB) of the Institute of Health of Jimma University with a reference number of IRB000/01/2020 before the commencement of the study. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study was based on anonymized patient records without contacting patients since the study was retrospective (conducted through patient chart review), which was waived by the IRB. However, after explaining the aim of the study, informed written consent was obtained from Jimma University Medical Center’s medical director on behalf of the patients to get full access to patient information and medical records. To ensure confidentiality, identifiers of preterm neonates and healthcare providers who examined the neonate were not recorded on the data collection checklist. All data collected from the respondents’ records were kept confidential by locking it with a password. After completing data entry, filled checklists were locked on the shelf. All study procedures followed the Declaration of the Helsinki Convention.
Author contributions
TT conceived the study and contributed to the work in study design, execution, data analysis, interpretation, report writing, drafting, and revising of the manuscript. HM and LD were involved in the conception, design, data analysis, and revising of the manuscript. All authors contributed to data analysis, drafting, and revising the paper and agreed to be accountable for all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank all neonatal intensive care unit and medical record staff members of Jimma University Medical Center for their cooperation and for providing the necessary information. We would also like to thank data collectors and supervisors.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2024.1606897/full#supplementary-material
A flow chart of selecting preterm neonates admitted to the NICU of JUMC, Southwest Ethiopia, 2020.
References
1.
March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. Eds CP Howson, MV Kinney, JE Lawn. Geneva: World Health Organization (2012).
2.
WHO. WHO Fact Sheet on Preterm Birth. Geneva: WHO (2018). Available from: http://www.who.int/mediacentre/factsheets/fs363/en. (Accessed December 24, 2021).
3.
United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). Levels & Trends in Child Mortality: Report 2018, Estimates Developed by the UN Inter-Agency Group for Child Mortality Estimation. New York: United Nations Children’s Fund (2018).
4.
WHO and UNICEF. Every Newborn Progress Report 2015. Geneva: World Health Organization (2015).
5.
Chawanpaiboon S Vogel JP Moller AB Lumbiganon P Petzold M Hogan D et al Global, Regional, and National Estimates of Levels of Preterm Birth in 2014: A Systematic Review and Modelling Analysis. Lancet Glob Health (2019) 7(1):e37–e46. 10.1016/S2214-109X(18)30451-0
6.
UNICEF. Every Child Alive: The Urgent Need to End Newborn Deaths. Genève, Switzerland: United Nations Children’s Fund (2018).
7.
Liu L Oza S Hogan D Chu Y Perin J Zhu J et al Global, Regional, and National Causes of Under-5 Mortality in 2000–15: An Updated Systematic Analysis With Implications for the Sustainable Development Goals. The Lancet (2016) 388(10063):3027–35. 10.1016/S0140-6736(16)31593-8
8.
Holditch-Davis D Santos H Levy J White-Traut R O'Shea TM Geraldo V et al Patterns of Psychological Distress in Mothers of Preterm Infants. Infant Behav Dev (2015) 41:154–63. 10.1016/j.infbeh.2015.10.004
9.
Trumello C Candelori C Cofini M Cimino S Cerniglia L Paciello M et al Mothers' Depression, Anxiety, and Mental Representations After Preterm Birth: A Study During the Infant's Hospitalization in a Neonatal Intensive Care Unit. Front Public Health (2018) 6:359. 10.3389/fpubh.2018.00359
10.
Jacob J Lehne M Mischker A Klinger N Zickermann C Walker J . Cost Effects of Preterm Birth: A Comparison of Health Care Costs Associated With Early Preterm, Late Preterm, and Full-Term Birth in the First 3 Years After Birth. Eur J Health Econ (2017) 18(8):1041–6. 10.1007/s10198-016-0850-x
11.
Opio C Malumba R Kagaayi J Ajumobi O Kamya C Mukose A et al Survival Time and its Predictors in Preterm Infants in the Post-Discharge Neonatal Period: A Prospective Cohort Study in Busoga Region, Uganda. Lancet Glob Health (2020) 8:S6. 10.1016/s2214-109x(20)30147-9
12.
Birhanu D Gebremichael B Tesfaye T Tadesse M Belege F Godie Y et al Survival Status and Predictors of Mortality Among Preterm Neonates Admitted to Neonatal Intensive Care Unit of Addis Ababa Public Hospitals, Ethiopia, 2021. A Prospective Cohort Study. BMC Pediatr (2022) 22(1):153. 10.1186/s12887-022-03176-7
13.
Bereka B Demeke T Fenta B Dagnaw Y . Survival Status and Predictors of Mortality Among Preterm Neonates Admitted to Mizan Tepi University Teaching Hospital, South West Ethiopia. Pediatr Health Med Ther (2021) 12:439–49. 10.2147/PHMT.S319774
14.
Girma B Berhe H Mekonnen F Nigussie J . Survival and Predictors of Mortality Among Preterm Neonates in Northern Ethiopia: A Retrospective Follow-Up Study. Front Pediatr (2023) 10:1083749. 10.3389/fped.2022.1083749
15.
Yismaw AE Gelagay AA Sisay MM . Survival and Predictors Among Preterm Neonates Admitted at University of Gondar Comprehensive Specialized Hospital Neonatal Intensive Care Unit, Northwest Ethiopia. Ital J Pediatr (2019) 45(1):4. 10.1186/s13052-018-0597-3
16.
Gebreheat G Teame H . Survival and Mortality of Preterm Neonates in a Neonatal Intensive Care Unit in Northern Ethiopia: A Retrospective Cohort Study. Sci Rep (2022) 12(1):600. 10.1038/s41598-021-04521-z
17.
Huka AE Oljira L Weldesenbet AB Bushra AA Ahmed IA Tura AK et al Predictors of Time to Death Among Preterm Neonates Admitted to Neonatal Intensive Care Units at Public Hospitals in Southern Ethiopia: A Cohort Study. PLoS One (2023) 18(10):e0283143. 10.1371/journal.pone.0283143
18.
Belay DM Worku WZ Wondim A Hailemeskel HS Bayih WA . Predictors of Survival Among Preterm Neonates Admitted to Felege Hiwot Comprehensive Specialized Hospital, Northwest Ethiopia. Front Pediatr (2022) 10:800300. 10.3389/fped.2022.800300
19.
USAID, PCI, GAPPS, ACNM. EVERY PREEMIE SCALE: Ethiopian Profile of Preterm and Low Birth Weight Prevention and Care (2019).
20.
Temesgen M Worku B Regassa Y Mekasha A . Survival of Preterm Infants Admitted to Tikur Anbessa Hospital Nicu, Addis Ababa. Ethiopian J Pediatr Child Health (2014) 10(1):68–78. Available from: https://www.ejpch.net/index.php/ejpch/article/view/109 (Accessed: December 24, 2022).
21.
FMOH. National Strategy for Newborn and Child Survival in Ethiopia, 2015/16-2019/20. Addis Ababa, Ethiopia: Maternal and Child Health Directorate. Addis Ababa, Ethiopia: Federal Ministry of Health (2015).
22.
Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF. Ethiopia Mini-Demographic and Health Survey 2019: Key Indicators Rockville. Maryland, USA: EPHI and ICF (2019).
23.
FMOH. Health and Health-Related Indicators. 2nd ed. Ethiopia: Federal Ministry of Health (2014).
24.
Child EWE. The Global Strategy for Women's, Children's and Adolescents' Health(2016-2030). Geneva (2015).
25.
FMOH. Neonatal Intensive Care Unit (NICU) Training Participants’ Manual. Addis Ababa, Ethiopia: Federal Ministry of Health (2021).
26.
VON. Global Neonatal Database Manual of Operations: Ethiopian Neonatal Network (ENN). Addis Ababa, Ethiopia: Vermont Oxford Network (2018).
27.
Mengesha HG Wuneh AD Lerebo WT Tekle TH . Survival of Neonates and Predictors of Their Mortality in Tigray Region, Northern Ethiopia: Prospective Cohort Study. BMC Pregnancy Childbirth (2016) 16(1):202. 10.1186/s12884-016-0994-9
28.
Wesenu M Kulkarni S Tilahun T . Modeling Determinants of Time-To-Death in Premature Infants Admitted to Neonatal Intensive Care Unit in Jimma University Specialized Hospital. Ann Data Sci (2017) 4(3):361–81. 10.1007/s40745-017-0107-2
29.
Bako B Idrisa A Garba MA Pius S Obetta HI . Determinants of Neonatal Survival Following Preterm Delivery at the University of Maiduguri Teaching Hospital, Maiduguri, Nigeria. Trop J Obstet Gynaecol (2017) 34(1):39–44. 10.4103/tjog.tjog_49_16
30.
Dagnachew T Yigeremu M . Survival of Preterm Neonates and its Determinants in Teaching Hospitals of Addis Ababa University. J Women's Health Care (2019) 8(2). 10.35248/2167-0420.19.8.461
31.
Schindler T Koller-Smith L Lui K Bajuk B Bolisetty S New South W et al Causes of Death in Very Preterm Infants Cared for in Neonatal Intensive Care Units: A Population-Based Retrospective Cohort Study. BMC Pediatr (2017) 17(1):59. 10.1186/s12887-017-0810-3
32.
Xu F Kong X Duan S Lv H Ju R Li Z et al Care Practices, Morbidity and Mortality of Preterm Neonates in China, 2013-2014: A Retrospective Study. Sci Rep (2019) 9(1):19863. 10.1038/s41598-019-56101-x
33.
Jain K Sankar MJ Nangia S Ballambattu VB Sundaram V Ramji S et al Causes of Death in Preterm Neonates (<33 Weeks) Born in Tertiary Care Hospitals in India: Analysis of Three Large Prospective Multicentric Cohorts. J Perinatology: official J Calif Perinatal Assoc (2019) 39(Suppl. 1):13–9. 10.1038/s41372-019-0471-1
34.
Travers CP Clark RH Spitzer AR Das A Garite TJ Carlo WA . Exposure to Any Antenatal Corticosteroids and Outcomes in Preterm Infants by Gestational Age: Prospective Cohort Study. BMJ (2017) 356:j1039. 10.1136/bmj.j1039
35.
McGoldrick E Stewart F Parker R Dalziel SR . Antenatal Corticosteroids for Accelerating Fetal Lung Maturation for Women at Risk of Preterm Birth. Cochrane Database Syst Rev (2020) 12(12):CD004454. 10.1002/14651858.CD004454.pub4
36.
Muhe LM McClure EM Nigussie AK Mekasha A Worku B Worku A et al Major Causes of Death in Preterm Infants in Selected Hospitals in Ethiopia (SIP): A Prospective, Cross-Sectional, Observational Study. Lancet Glob Health (2019) 7(8):e1130–8. 10.1016/S2214-109X(19)30220-7
37.
Collins A Weitkamp JH Wynn JL . Why Are Preterm Newborns at Increased Risk of Infection?Arch Dis Child Fetal Neonatal Ed (2018) 103(4):F391–F394. 10.1136/archdischild-2017-313595
38.
Pratiwi SR Prasetya H Murti B . The Effect of Asphyxia on Neonatal Death: A Meta-Analysis. J Matern Child Health (2021) 5(4):413–21. 10.26911/thejmch.2020.05.04.08
39.
Mekonnen AG Yehualashet SS Bayleyegn AD . The Effects of Kangaroo Mother Care on the Time to Breastfeeding Initiation Among Preterm and LBW Infants: A Meta-Analysis of Published Studies. Int Breastfeed J (2019) 14:12. 10.1186/s13006-019-0206-0
40.
Uwaezuoke SN . Kangaroo Mother Care in Resource-Limited Settings: Implementation, Health Benefits, and Cost-Effectiveness. Res Rep Neonatal (2017) 7:11–8. 10.2147/rrn.s138773
Summary
Keywords
incidence, mortality, preterm, neonate, predictors
Citation
Toma TM, Merga H and Dube L (2024) Incidence and Predictors of Mortality Among Preterm Neonates Admitted to Jimma University Medical Center, Southwest Ethiopia: a Retrospective Follow-Up Study. Int J Public Health 69:1606897. doi: 10.3389/ijph.2024.1606897
Received
25 November 2023
Accepted
20 June 2024
Published
04 July 2024
Volume
69 - 2024
Edited by
Jean Tenena Coulibaly, Félix Houphouët-Boigny University, Côte d’Ivoire
Reviewed by
Ramesh Poluru, INCLEN Trust, India
Hongzhao You, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Tigabu Kidie Tesfie, Debre Markos University, Ethiopia
One reviewer who chose to remain anonymous
Updates
Copyright
© 2024 Toma, Merga and Dube.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Temesgen Mohammed Toma, tememohamme@gmail.com
ORCID: Temesgen Mohammed Toma, orcid.org/0000-0001-8849-6722; Hailu Merga, orcid.org/0000-0001-7536-4755
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.