- 1Institute for Human Development, Aga Khan University, Nairobi, Kenya
- 2Centre for Geographic Medicine Research Coast, Kenya Medical Research Institute (KEMRI), Kilifi, Kenya
- 3School of Public Health, University of the Witwatersrand, Johannesburg, South Africa
- 4MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- 5Department of Psychiatry, Warneford Hospital, University of Oxford, Oxford, United Kingdom
- 6Department of Public Health, Pwani University, Kilifi, Kenya
Objectives: a) To document the prevalence and correlates of frailty among older adults living with HIV (OALWH) and their uninfected peers, and b) Investigate HIV status as an independent predictor of frailty.
Methods: This cross-sectional study was conducted between 2020 and 2021 at the Kenyan coast among 440 older adults aged ≥50 years (257 OALWH). Frailty was assessed using the Reported Edmonton Frail Scale. Logistic regression was used to examine the correlates of frailty.
Results: The prevalence of frailty was significantly higher among OALWH (24%) than their uninfected peers (13%). HIV seropositivity was not independently associated with frailty. Sleeping difficulties, ageism, higher waist/hip ratio, visiting traditional healers, HIV treatment change/interruption, prolonged illness following HIV diagnosis, and self-reported diabetes were significantly associated with higher odds of frailty. Residing in larger households, having higher income, having friends, being male and light physical activities were significantly associated with reduced odds of frailty.
Conclusion: The prevalence of frailty is elevated among OALWH; however, factors other than HIV are predominant, particularly psychosocial factors. Multicomponent interventions are needed to prevent/delay and manage frailty in this setting.
Introduction
Recent years have witnessed a remarkable rise in the population of older adults in developing countries, including Kenya, making caring for the elderly a public health priority [1]. A better understanding of the factors that influence healthy ageing is crucial for designing appropriate therapies to prevent functional decline, maintain independence, and preserve the quality of life of these adults. In this context, increasing attention has been paid to geriatric syndromes, especially frailty, as a potential explanation of the health diversity among older adults [2]. Although a universal definition is still lacking, frailty is commonly conceptualized as the increased vulnerability to external and internal stressors resulting from a significant loss of physiologic reserve [3]. Several pathophysiological pathways, including cellular senescence, mitochondrial dysfunction, oxidative stress, and dysregulation of inflammatory processes, underlie the frailty syndrome [4]. Growing evidence from systematic reviews also links frailty with adverse health outcomes, including emergency hospital admissions [5], disability [6], poor quality of life [7], dementia [8], and premature mortality [9]. Given the high burden, negative impacts on older adults, and the dynamic nature of frailty, identifying the determinants of frailty is imperative, especially among vulnerable older adults such as those ageing with HIV.
Frailty has been examined extensively in recent years; however, most work has been conducted in older populations in high-income countries (HICs). Some work has been conducted in LMICs, but this has been confined to Brazil, Mexico, China [10] and South Africa [11–14]. So far, results indicate that the prevalence of frailty is highly variable, both between countries and between populations within a country, due to differences in the populations studied and the measurement of frailty. A recent meta-analysis of European studies showed a frailty prevalence of 12% among community-based studies and 45% in non-community-based studies [15]. In Latin America, the prevalence of frailty among community-dwelling older adults was 20%, with a range of 8%–43% in the studies reviewed [16]. Among OALWH, the global prevalence of frailty ranges from 5% to 29% [17]. In SSA, there has been growing attention to the risks of frailty in the ageing population, both in the general population and among people living with HIV (PLWH). To our knowledge, about a dozen studies on frailty have been conducted among older adults in the general population in SSA: South Africa [11–14], Tanzania [18–20], Ghana [13, 14], Nigeria [21], and Burkina Faso [22] with prevalence estimates ranging from 5.4% [11] to 63.3% [21]. Only two studies have focused on OALWH [23], with prevalence estimates ranging from 2.8% to 14.7%.
Identifying the risk and protective factors of frailty may be useful for developing interventions designed to prevent and/or lower the burden that frailty places on a person and provide future directions for public health policy. Indeed, numerous studies have focused on identifying the factors associated with frailty, including the role of biological, lifestyle, and psychological factors [18]. Among older adults in the general population, sociodemographic factors (e.g., being female, older age, low education levels, low income, living alone), physical factors (e.g., obesity, malnourishment, hearing loss, visual impairment, persistent pain), lifestyle factors (e.g., smoking, alcohol consumption, sedentary behaviours) and psychological factors (e.g., depressive symptoms, and sleeping problems) have been identified as risk factors for frailty [24–33]. Most of these studies have focused on sociodemographic factors. However, recently published studies have focused more on lifestyle-related, psychological, and biological factors associated with frailty, which may reflect a growing interest in potentially modifiable factors for frailty. Among OALWH, less research on risk and protective factors has been conducted. In a past systematic review [17], the predictors of frailty included older age, comorbidities, diagnosis of acquired immunodeficiency syndrome (AIDS) and low current CD4+ cell count.
Like many parts of SSA, Kenya’s population of older adults is increasing rapidly. In the most recent population census of 2019, the proportion of older adults aged ≥50 years was about 11%, representing approximately 5.2 million individuals [34]. Kenya is also witnessing an increase in chronic age-related conditions [35], which coincides with a high prevalence of HIV among those aged ≥50 years [36]. Hence, establishing the current burden and determinants of frailty is essential if health and social care services are to meet the needs of Kenya’s ageing population. The present study aims to: a) determine the prevalence of frailty among OALWH compared to their uninfected peers; b) investigate HIV status as an independent predictor of frailty in the older adults; and c) investigate the determinants of frailty among the older adults at the coast of Kenya.
Methods
Study Design and Setting
This was a cross-sectional study carried out at the Kenyan coast in Mombasa and Kilifi counties between 2020 and 2021. With an estimated population of about 1.5 million people [37], most Kilifi residents are rural inhabitants of the Mijikenda tribe, whose primary source of livelihood is subsistence farming and small-scale trading. In Kilifi, the prevalence of HIV in adults is 4.5% [38]. Mombasa County borders Kilifi to the north and hosts Mombasa City, the second-largest city and chief port of Kenya. It has a population of about 1.2 million residents [37]. Given its urban nature, the county is made up of the local (Mijikenda and Swahili) and immigrant communities from other parts of Kenya. At about 60%, the formal sector provides the majority of employment in Mombasa County [39]. The prevalence of HIV in adults in Mombasa is about 7.5% [38].
Study Participants and Recruitment
Older Adults Living With HIV (OALWH)
We recruited the OALWH from two public HIV-specialized clinics in Mombasa and Kilifi counties (one in each). We specifically selected the two clinics because of their wide client catchment area and their large volume of potential participants. To be included, clients had to be aged ≥50 years of age, have a confirmed HIV seropositivity status, be on HIV treatment, and be willing and able to provide informed consent for their involvement.
In both HIV clinics, two community health volunteers or healthcare providers assisted us in reviewing existing records to identify potential clients. Efforts were made to contact all potential clients who had contact details (in alphabetical order) to invite them to participate in our study. Study introductions were conducted in person by a research assistant before any enrolment. Participant recruitment began in Mombasa County; however, it was interrupted by the onset of the COVID-19 pandemic after recruiting and assessing only 72 OALWH. Upon resumption of project activities, the recruitment and assessment of remaining clients (n = 368) took place in Kilifi County.
HIV Uninfected Older Adults
All the older adults without HIV were recruited from Kilifi County. The Kilifi Health and Demographic Surveillance System (KHDSS) was used to identify families with eligible older adults. Subsequently, potential participants aged ≥50 years were randomly identified from the existing database and followed up at their homes using Global Positioning System (GPS) coordinates by our trained research assistants. Project information was shared with all individuals who expressed interest in participation. As inclusion criteria, individuals had to be ≥50 years old, inhabitants of Kilifi county, and provide consent to their involvement, including willingness to be tested for HIV using a rapid HIV testing kit (OraQuick) for a confirmation of their HIV seronegative status.
Sample Size Calculations
We calculated our sample size using a previous study [40], which reported significant differences in the prevalence estimate of frailty between OALWH and their uninfected counterparts. Power analyses in Stata (using effect estimates and comparison of two group proportions) were conducted to estimate the required sample size. An overall sample of 310 was required to detect a difference in frailty between OALWH and uninfected peers at 80% power and a 5% level of statistical significance. A sample of 450 participants was deemed sufficient, allowing for missing data.
Measures
We programmed all our research instruments on Android tablets using the Research Electronic Data Capture (REDCap) platform [41] for face-to-face interviewer administration. The first author (the study coordinator) trained the research assistants for 2 weeks to facilitate the proper administration of the study tools. All study tools not previously adapted to the local language of Swahili underwent recommended adaptation procedures, that is, forward translation, forward translation review, back translation, harmonization by a panel of experts, pilot testing, pilot testing review, and proofreading [42].
Sociodemographic and Asset Index Form
Sociodemographic characteristics, including participants’ age, sex, marital status, educational level, occupational status, household size, income, living arrangements and number of dependents, were captured in REDCap. We also collected information on individual and family ownership of disposable assets for asset index computation as a proxy for socioeconomic status. The participants also provided information on their food security in the past week, access to social support, social network of close friends, the number of people living with HIV in the household, and whether they were taking care of sick family members at the time and visiting traditional healers.
General Health Information
We also gathered the participants’ anthropometric details (such as height, weight, blood pressure, waist, and hip circumference), hours spent on sedentary activities in a day, sexual activity, number of medications one was using, self-reported comorbidities, past medical history, and common complaints, e.g., fatigue, pain, sleeping difficulties, visual and hearing problems.
For OALWH, we also asked HIV-specific questions relating to the disclosure of HIV status, access to the HIV clinic, and past medical history, e.g., cART regimen change/interruption and prolonged illness after HIV diagnosis. Information pertaining to their current ART regimen and overall duration on ART were extracted from their medical records. We also collected 10 mL of venous blood samples from the OALWH for viral load measurement.
Psychosocial Measures
Psychosocial variables included HIV-related stigma, functional disability, loneliness, and age-related discrimination (ageism). All these constructs were assessed using interviewer-administered Likert scales, the brief 12-item HIV stigma scale [43], the 12-item World Health Organization Disability Assessment Schedule 2 [44], UCLA 8-item loneliness scale [45], and the 20-item ageism survey [46]. In each scale, a higher score translates into a greater level of impairment.
Measures of Frailty
We assessed frailty using the modified Reported Edmonton Frail Scale [47]. It assesses nine domains of frailty: cognition, general health status, functional independence, social support, medication use, nutrition, mood, continence, and self-reported functional performance. Each domain comprises a set of questions examining the construct. Test scores range from 0–18, and participants are classified conventionally into 5 categories, with a higher score representing a higher degree of frailty: 0–5 (not frail), 6–7 (apparently vulnerable), 8–9 (mild frailty), 10–11 (moderate frailty) and 12–18 (severe frailty). In the current study, we collapsed these categories into three: 0–5 (non-frail), 6–7 (pre-frail) and 8–18 (frail) to enable meaningful analysis of the correlates of frailty [48, 49].
Data Analysis
We conducted all our analyses in STATA version 15.0 (StataCorp LP, College Station, TX, United States). We utilized descriptive statistics to summarize sample characteristics. Specifically, independent Student’s t-test and Chi-square test were used to compare differences in independent variables. Proportions were used to estimate the prevalence of frailty among OALHW and their uninfected peers. To examine HIV status as an independent predictor of frailty, we used logistic regression analyses adjusting for relevant exposure variables that accounted for differences in frailty. Examination of the correlates of frailty applied logistic regression models to explore univariate associations between the binary outcome variables (frailty) and the various exposure variables. Expose variables with a p-value <0.15 in the univariate analysis were then entered into the multivariable models using forward selection. In all models, collinearity was checked, and for all hypothesis tests, a two-tailed p-value <0.05 was deemed statistically significant. We checked the overall fit of the final models using Hosmer and Lemeshow’s goodness of fit, where a p-value of >0.05 was considered a good fit.
Results
Sample Characteristics
Our sample comprised 440 participants, 257 (58%) of whom were OALWH. An overall response rate of 90% was achieved at recruitment. Among the respondents, 6 (1.4%) did not complete the outcome measure. Table 1 gives the details of the demographic and biopsychosocial information of these participants. In brief, the mean age of the respondents was 60.1 (SD = 6.9) years and 58.6% were female. The majority of the participants were unemployed (65.5%), had a monthly household income of less than 10,000 Kenyan shillings—about $90 (63.4%), lived in multigenerational households (81.6%), and had caregiving responsibilities (66.4%). Moreover, close to half of them reported being sexually active, and a similar proportion reported that they were not accessing/receiving adequate social support. Adults living with HIV were likely to be younger, unmarried, more educated, have lower monthly household income, live alone, and have fewer dependents.
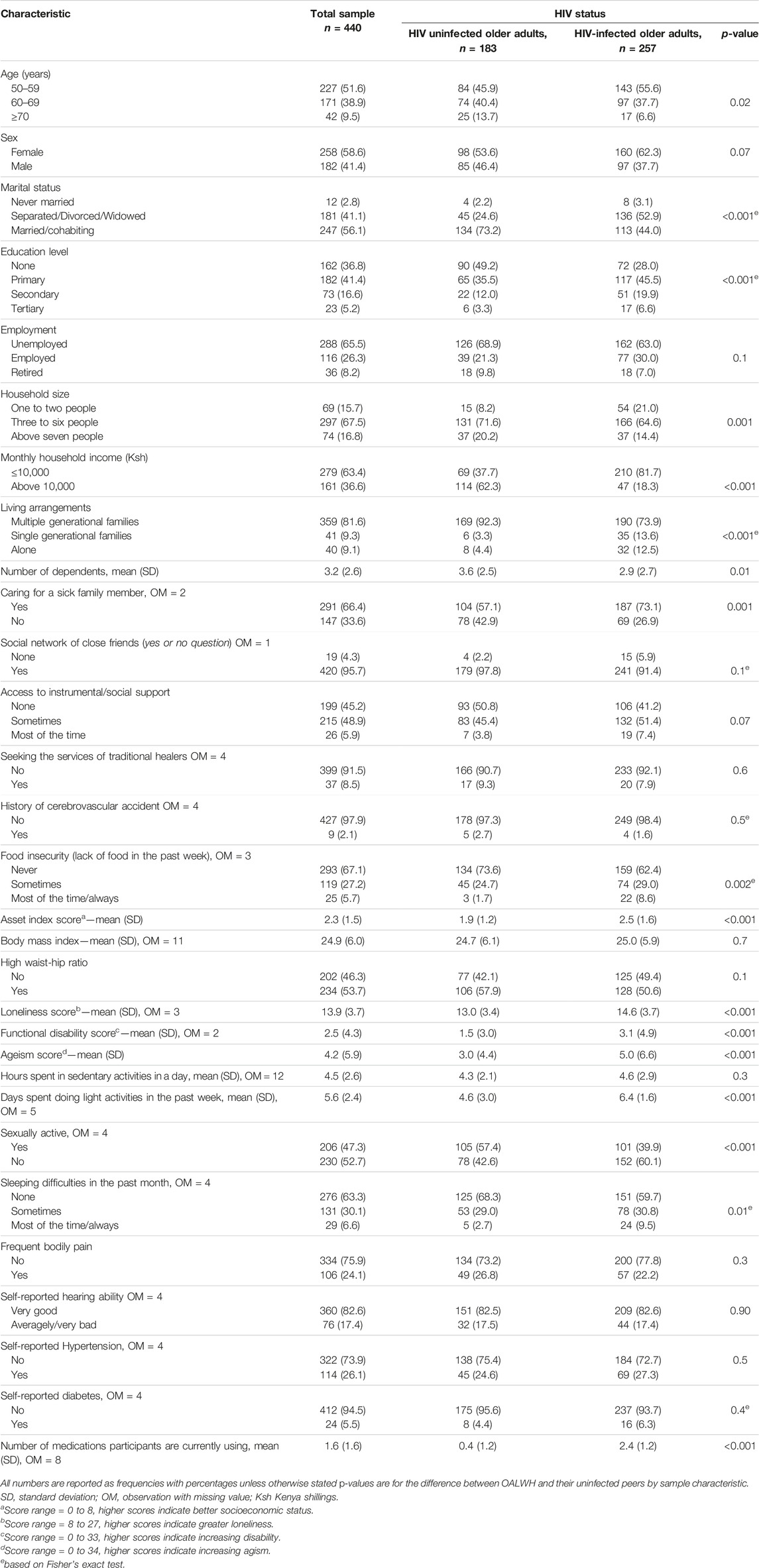
TABLE 1. Characteristics of the study population by HIV status, n = 440 (The HIV-associated Neurocognitive Disorders study, Kenya, 2020 and 2021).
HIV-Related Characteristics of Older Adults Living With HIV
All the OALWH were receiving HIV treatment, most (90%) of whom were on a first-line cART regimen. Most (95.3%) of them had disclosed their HIV status. The mean (SD) duration of HIV treatment was 11.4 (4.3) years. Additionally, nearly all of them (98.1%) had suppressed viral load (≤1,000 copies/mL). Further details are highlighted in Table 2.
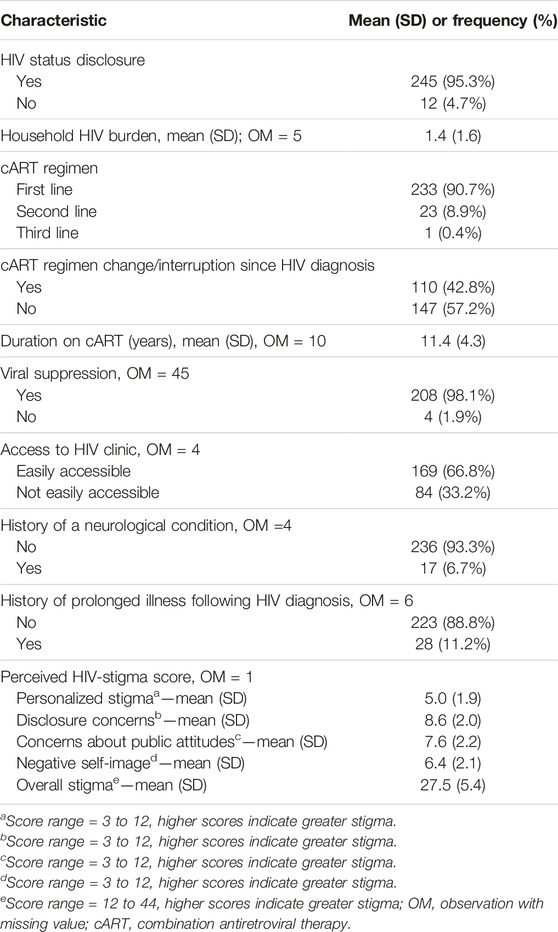
TABLE 2. HIV-related, clinical, and psychosocial characteristics of OALWH, n = 257 (The HIV-associated Neurocognitive Disorders study, Kenya, 2020 and 2021).
Frailty Prevalence Estimates
The overall prevalence of frailty across the sample was 19.4% (95% CI: 15.7–23.4). Groupwise, older adults living with HIV presented with a significantly higher prevalence of frailty (23.9%) than their uninfected peers (12.8%), p < 0.01 (Table 3).
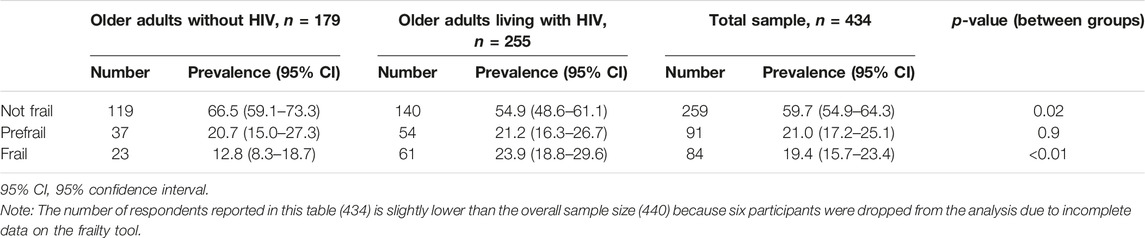
TABLE 3. Prevalence of frailty in OALWH versus their uninfected peers (The HIV-associated Neurocognitive Disorders study, Kenya, 2020 and 2021).
Association Between HIV Status and Frailty
In univariate logistic regression analyses (Table 4), HIV seropositivity was significantly associated with higher odds of frailty (OR 2.13; 95% CI 1.26, 3.60). However, in the multivariable logistic regression model (Table 4), HIV seropositivity was not significantly associated with frailty (aOR 1.26; 95% CI 0.60, 2.63).
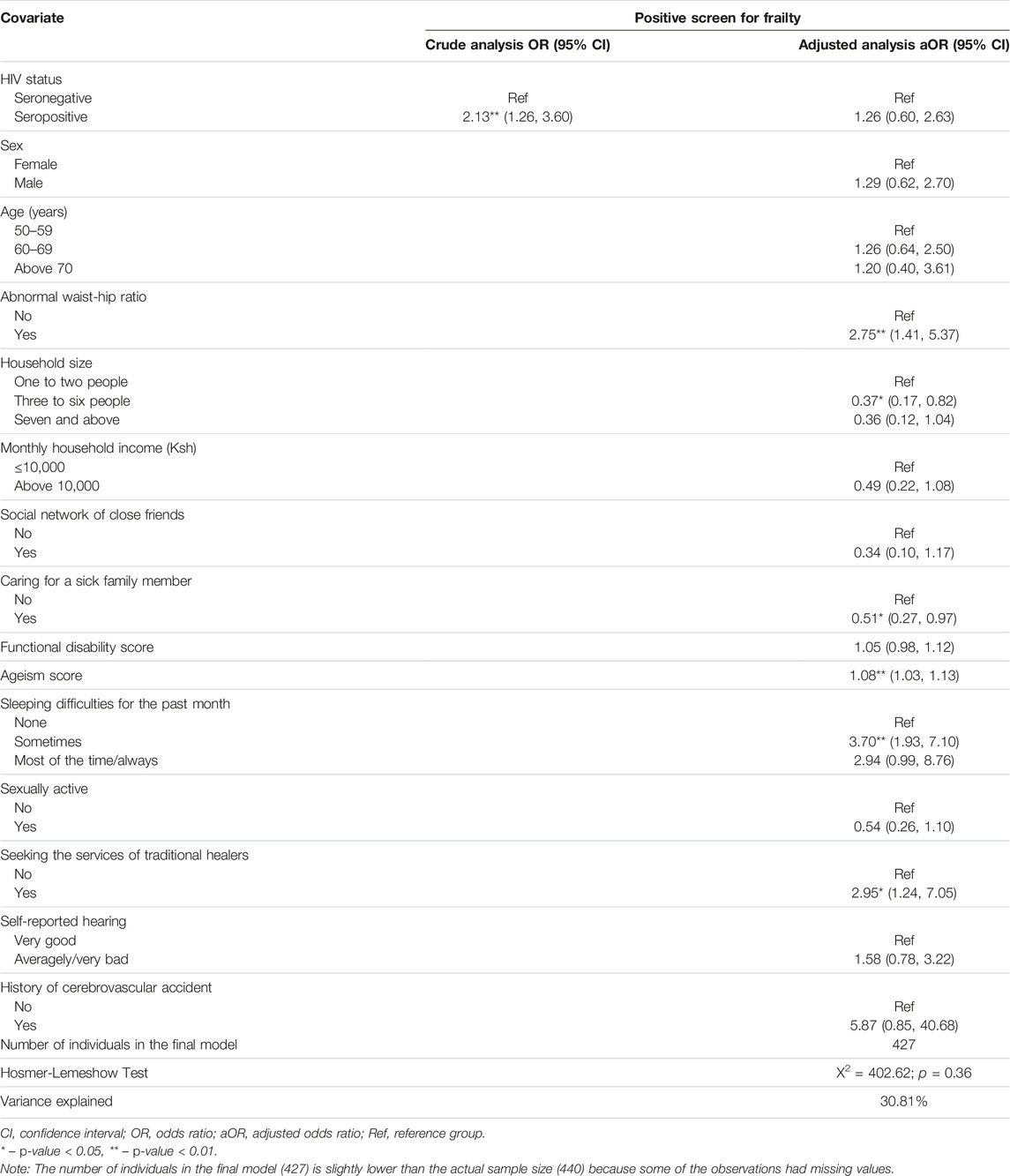
TABLE 4. Association between HIV status and frailty among older adults (The HIV-associated Neurocognitive Disorders study, Kenya, 2020 and 2021).
Determinants of Frailty in Older Adults Living With HIV
Table 5 presents results from univariate and multivariate logistic regression analyses exploring the determinants of frailty among older adults living with HIV.
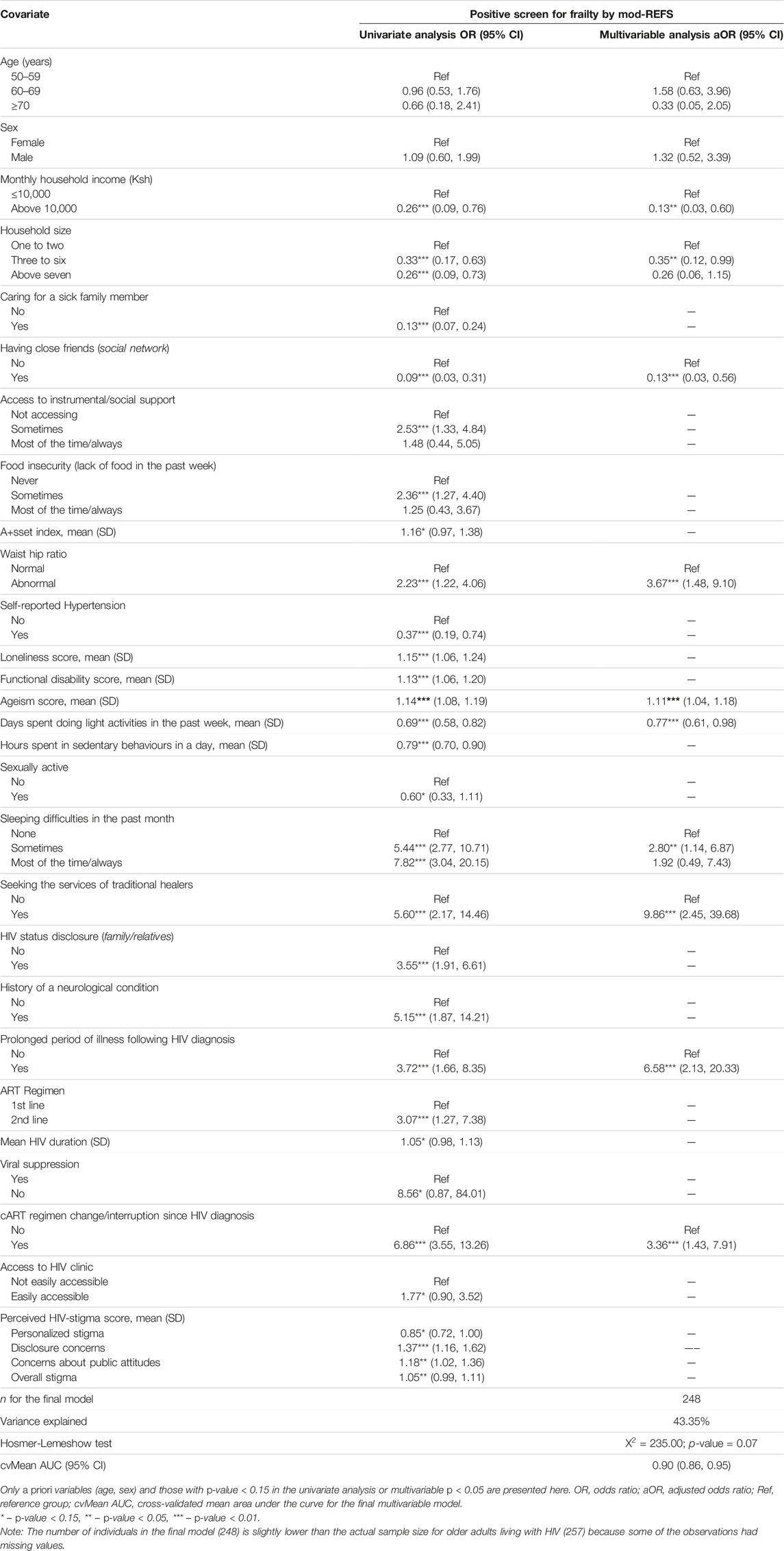
TABLE 5. Univariate and multivariable analysis of correlates of frailty among OALWH (The HIV-associated Neurocognitive Disorders study, Kenya, 2020 and 2021).
In the multivariable logistic regression model, factors significantly associated with higher odds of frailty among OALWH were sleeping difficulties in the past month, increasing ageism scores, visiting traditional healers, high waist-to-hip ratio, a history of cART regimen change/interruption, and a history of prolonged illness after HIV diagnosis. On the other hand, having a social network of close friends, a larger household, a higher household income (≥10,000 Ksh; about $86) and taking part in light physical activities such as walking in the past week were significantly associated with lower odds of frailty.
Determinants of Frailty in Older Adults Without HIV
In multivariable analyses (Table 6), sleeping difficulties in the past month, self-reported diabetes, and light physical activities in the past week were significantly associated with higher odds of frailty among the HIV uninfected older adults. Conversely, being male was significantly associated with reduced odds of frailty in these adults.
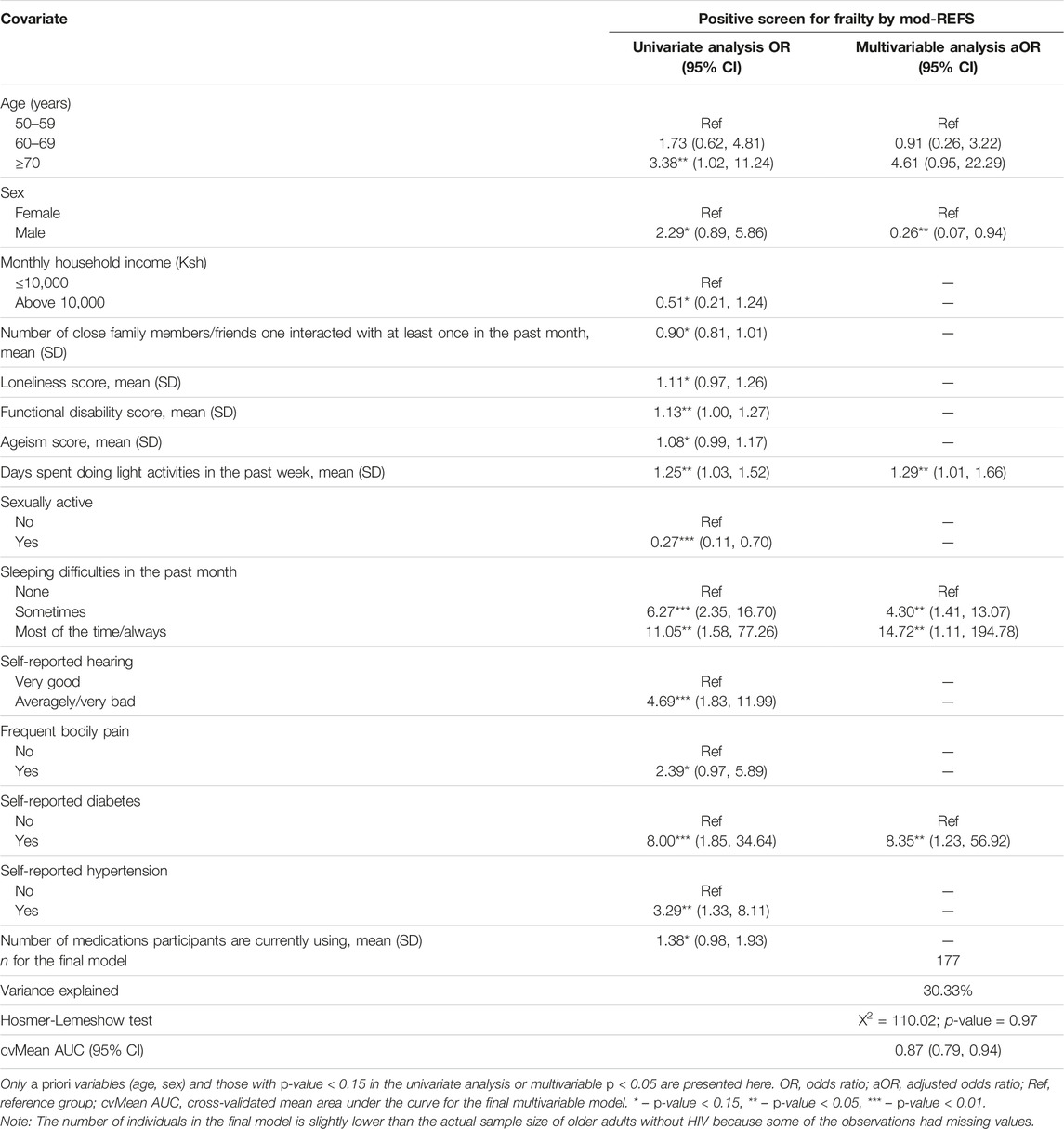
TABLE 6. Univariate and multivariable analysis of correlates of frailty among HIV-uninfected older adults (The HIV-associated Neurocognitive Disorders study, Kenya, 2020 and 2021).
Discussion
Our study adds to the growing number of reports on the burden and determinants of frailty in low- and middle-income countries. In this study, the prevalence of frailty was significantly higher in OALWH (23.9%; 95% CI 18.8–29.6) compared to their uninfected peers (12.8%; 95% CI 8.3–18.7); however, HIV seropositivity was not significantly associated with frailty after adjusting for biopsychosocial factors. To our knowledge, there have been only two previous studies of frailty among OALWH in SSA. The first one, a cross-sectional sample of 145 OALWH (67% female) on ART and a median age of 57 years in Tanzania, reported a low prevalence of frailty (2.8%) [50]. The other one, a population-based cohort of 614 older adults (292 OALWH) in South Africa, reported a frailty prevalence of 17.7% in OALWH compared to 14.7% in their uninfected peers [51]. The variations in frailty prevalence could partly be attributed to differences in frailty measurement and the fact that older adults are a highly heterogeneous group, having different genetic backgrounds, medical profiles, and biological, as well as social-environmental factors at different stages of life, thus highlighting the need for country-specific frailty data using tools validated within the country or region of interest. Our finding of a higher prevalence of frailty in OALWH than their uninfected peers is consistent with previous evidence, which has demonstrated both higher frequency of frailty in OALWH and the development of frailty at earlier ages for OALWH than for individuals without HIV [17]. Many factors may predispose OALWH to elevated rates of frailty, including the effects of persistent inflammation from HIV (even in well-controlled viraemia), toxic effects of earlier cART regimens, delayed initiation of ART, and higher rates of multimorbidity [52].
Literature suggests that it is a constellation of environmental, disease-specific, and biological factors that contribute to frailty [17, 24–33], though most of the evidence is concentrated in HICs. In the present study, the factors significantly associated with frailty were predominantly psychosocial, many of which are potentially modifiable with appropriate programs and interventions. Consistent with previous studies [33, 53], our study showed that sleeping difficulties were associated with higher odds of frailty in OALWH and their uninfected peers. Sleep problems may impact frailty in numerous ways, including decreased energy expenditure, elevated inflammatory response, disturbed hormonal pathways, tissue growth and repair [53]. These mechanisms may explain the associations observed. Interventions targeting sleeping problems—such as exercise and mindfulness-based stress reduction, may have potential clinical implications for OALWH and their uninfected peers.
Increasing ageism scores were also significantly associated with elevated odds of frailty in OALWH in our study. Ageism, commonly conceptualized as the stereotyping, prejudice and discrimination against people based on age, is becoming increasingly important in older adults [54]. Very few studies have examined the possible association between frailty and ageism. Our finding is consistent with the limited previous research [55]. Persistent exposure to ageism could lead to the internalization of the ageist messages by the OALWH, thus becoming part of their unconscious beliefs. In some cases, the adults may act subconsciously to fulfil the ageist stereotypes, even if detrimental to their health and wellbeing, e.g., physiologic stress response, and physical functioning performance, which may influence frailty. The observed association could also be explained by the health status and higher educational levels among OALWH.
A higher waist/hip ratio was also significantly associated with elevated odds of frailty among OALWH in our study, consistent with previous findings of a positive association between central/abdominal obesity and frailty [56, 57]. As HIV infection has become a manageable chronic illness, it has been progressively accompanied by a growing prevalence of overweight and obesity [58]. Moreover, long-term treatment with cART may contribute to lipodystrophy, often characterized by fat redistribution with a relative increase in abdominal fat [59]. Lipid depositions and infiltration in muscle fibre may bring about frailty by decreasing mobility and increasing loss of muscle strength. Interventions to minimize obesity and sedentary behaviour could potentially be beneficial in addressing frailty.
Visiting traditional healers was significantly associated with elevated odds of frailty among OALWH in our study. We are not aware of previous research that has examined this relationship. Plausibly, frail individuals visit the healers to have their frailty symptoms addressed, especially when the primary care services fail to address these concerns. A previous qualitative exploration of the health and wellbeing of OALWH in the study setting has linked seeking help or treatment from traditional and certain faith healers with poor health outcomes, including defaulting HIV treatment and unsuppressed viral load [60]. Healthcare providers have a reason to be concerned, given the observed impacts of untreated or improperly treated HIV on the development of frailty among OALWH.
Only two HIV-related factors were associated with elevated odds of frailty in our sample: a history of cART regimen change/interruption and prolonged illness following HIV diagnosis. These factors may be indicators of virological failure, ART toxicity or late HIV diagnosis, commonly associated with poorer health outcomes and risk of severe disease. Indeed, previous research has revealed an independent positive association between AIDS diagnosis, viral-load non-suppression, low CD4 count and frailty [17, 61]. Many of the OALWH who have lived with HIV for several years may be significantly impacted by the legacy of the early years of the epidemic, thus predisposing them to a heightened risk of frailty. These adults will more likely require additional support to manage the challenges of ageing with HIV successfully.
Several social factors, e.g., social isolation, social networks, socioeconomic status, social support, social engagement, and social capital, have the potential to influence the health of older adults [62]. In our study, higher monthly household income, residence in a larger household, and having a social network of close friends were all associated with reduced odds of frailty among OALWH, thus confirming previous research [17, 31] and highlighting the importance of promoting positive social factors to aid healthy ageing in older adults living with HIV. These factors may, directly and indirectly, affect frailty, e.g., food security, energy expenditure, and better health-seeking behaviours.
Physical activities have the potential to promote physical function, prevent falls and improve general health, hence delaying the onset and progression of frailty. In our study, participating in light physical activities such as walking was associated with reduced odds of frailty among OALWH, confirming previous research [63]. Exercise may also have positive effects on obesity, stress, loneliness in the case of group activities and muscular strength, which have been identified as potential risk factors for the incidence of frailty. However, light physical activities were associated with elevated odds of frailty among the HIV uninfected older adults in our study. This was a surprise finding, inconsistent with previous investigations [64–66]. This warrants more exploration in the study setting.
Self-reported diabetes was also associated with higher odds of frailty among HIV uninfected older adults in our study, consistent with previous reports [67]. Prospective evidence suggests that unhealthy behaviours and obesity may partly explain the association, and to a larger extent, by poor glucose control and altered serum lipid profile among individuals with diabetes, suggesting that diabetes nutritional therapy may reduce the risk of frailty [67]. Individuals with diabetic neuropathy are particularly at an increased risk of early-onset frailty [68]. Prevention programmes in the pre-frail states through appropriate exercise, nutrition and glycemic control may delay the development of frailty in these adults.
Similar to previous studies, this study showed that males are less likely to be frail than females, suggesting that being male is a protective factor against frailty [24]. Differences in physical activity, muscle mass and higher fat percentages may explain gender discrepancies in frailty. This may also be a question of selection—a classical observation in gerontological research where women live longer but in poorer health [69]. In this respect, men experience more life-threatening chronic conditions compared to women who experience more “non-life-threatening” conditions associated with more morbidity—as such the men who survive are those who tend to have better health status. Still, our observation may be related to social stigma of males appearing/acting/reporting weakness, that is, social preference bias, given the self-reported nature of the construct.
Implications
The prevalence of frailty in this study was relatively high for both OALWH and their uninfected peers. Preventing, delaying, or treating frailty is more critical in this setting, given its high burden and the fact that frailty is a known predictor of future disability and dependency. Within the clinical practice, an easy-to-use frailty score will allow the easy identification of those at risk, thus allowing planning of future health and social care needs of these adults. Our study also highlighted some of the correlates of frailty in this setting. Many of the factors identified, such as sleeping difficulties, social engagement, ageism, and visiting traditional healers, are potentially modifiable with appropriate programs. Our findings provide the foundation for developing culturally appropriate interventions and healthcare strategies to prevent, delay and manage frailty and its consequences to improve the health and functional status of older adults at risk of frailty. Individual, community-based, or clinic-based interventions such as comprehensive geriatric assessment, physical activity, promoting social engagement, addressing discrimination, and proper management of comorbidities, e.g., diabetes, may benefit older adults. Wider public health approaches, including proactive testing of older adults to avoid late diagnosis and advanced immunosuppression, will benefit OALWH. Our results also highlight the need for well-designed prospective studies to establish the incidence, pathophysiology, predictors of transition and outcomes of frailty in this population and assess potential interventions.
Strengths and Limitations
Our study is among the very few reports on frailty in SSA and the first one in Kenya. It extends the existing evidence base regarding the prevalence and correlates of frailty in low-resource settings like Kenya. A further strength is the use of a relatively large sample size of people ≥50 years living with HIV and the inclusion of a community-based comparison group which enabled us to give a detailed profile of frailty in this population. We also collected detailed information on sociodemographic, physical, lifestyle and psychological factors, which helped us examine the correlates of frailty in this population. The primary limitation, nonetheless, was the use of a cross-sectional design, which precludes any conclusions on causality. Besides, our sample was predominantly from a rural setting; thus, our participants’ experiences may differ from those in urban places. Also, the OALWH were invited to participate from a health facility (i.e., not a population-based sample) and may not be entirely representative of all OALWH in this area. For instance, the sample of OALWH in the study had very high levels of cART treatment and viral suppression, and our observations would probably be different in individuals not seeking care (either because they are unaware of their status or do not believe they need treatment).
Conclusion
In this cross-sectional study, we found a significantly higher prevalence of frailty among OALWH (24%) compared to their uninfected peers (13%). However, HIV seropositivity was not significantly associated with frailty after adjustment for demographic, psychosocial and physical factors, underscoring the importance of these factors. Specifically, OALWH who experience sleeping difficulties, ageism, have a high waist/hip ratio, visit traditional healers, have a history of cART regimen change/interruption and prolonged illness after HIV diagnosis have higher odds of frailty. In contrast, those who reside in larger households, have higher household income, have a social network of friends, and engage in light physical activity have reduced odds of frailty. On the other hand, HIV-uninfected older adults with sleeping difficulties, self-reported diabetes and taking part in light physical activities have elevated odds of frailty, while being male is associated with reduced odds of frailty. These factors should be considered in designing and implementing programs to prevent, delay, or treat frailty in this setting. Further prospective work is required to investigate the directionality and potential mediators of the association between frailty and the observed correlates.
Data Availability Statement
Application for data access can be made through the Data Governance Committee of the KEMRI Wellcome Trust Research Programme who will review the application and advise as appropriate, ensuring that uses are compatible with the consent obtained from participants for data collection. Requests can be sent to the coordinator of the Data Governance Committee using the following email, ZGdjQGtlbXJpLXdlbGxjb21lLm9yZw==.
Ethics Statement
The studies involving humans were approved by the Kenya Medical Research Institute Scientific and Ethics Review Unit (Ref: KEMRI/SERU/CGMR-C/152/3804). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author Contributions
PM, CRN, and AA conceptualized the study. PM, CRN, RW, and AA designed the study. PM and CN programmed the study questions on tablets and managed project data for the entire study period. PM analysed the data. PM, CN, RW, CRN, and AA contributed to the interpretation of the data. PM wrote the first draft of the manuscript, and all the authors reviewed the subsequent versions and approved the final draft for submission.
Funding
This work was funded by the Wellcome Trust International Master’s Fellowship to PM (Grant number 208283/Z/17/Z). Further funding supporting this work was from 1) the Medical Research Council (Grant number MR/M025454/1) to AA. This award is jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under MRC/DFID concordant agreement and is also part of the EDCTP2 program supported by the European Union; 2) DELTAS Africa Initiative [DEL-15-003]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [107769/Z/10/Z] and the UK government. The funders did not have a role in the design and conduct of the study or interpretation of study findings. RW is supported by the South African National Research Foundation (119234).
Author Disclaimer
The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Acknowledgments
An earlier version of this manuscript has been uploaded to Research Square preprint server ahead of peer review [70]. We would also like to thank all the participants who voluntarily participated in this study. We are grateful to the community health volunteers and healthcare providers at the HIV clinics for their overwhelming support during the study. We also acknowledge Sadaka Charo, Richard Karisa, Irene Kasichana, Maureen Nyadzua, Haprity Mwangata, Linda Moranga, Khamis Katana, Beatrice Kabunda, Katana Ngombo, Alfred Ngombo, Collins Kipkoech, and Martha Kombe for their role in data collection. This work is published with the permission of the director of the Kenya Medical Research Institute.
References
1. Nations, U. Department of Economic and Social Affairs, Population Division (2019). World Population Ageing 2019: Highlights (ST/ESA/SER. A/430).
2. Clegg, A, Young, J, Iliffe, S, Rikkert, MO, and Rockwood, K. Frailty in Elderly People. The lancet (2013) 381(9868):752–62. doi:10.1016/S0140-6736(12)62167-9
3. Sezgin, D, O’Donovan, M, Cornally, N, Liew, A, and O’Caoimh, R. Defining Frailty for Healthcare Practice and Research: A Qualitative Systematic Review With Thematic Analysis. Int J Nurs Stud (2019) 92:16–26. doi:10.1016/j.ijnurstu.2018.12.014
4. Viña, J, Tarazona-Santabalbina, FJ, Pérez-Ros, P, Martínez-Arnau, FM, Borras, C, Olaso-Gonzalez, G, et al. Biology of Frailty: Modulation of Ageing Genes and its Importance to Prevent Age-Associated Loss of Function. Mol aspects Med (2016) 50:88–108. doi:10.1016/j.mam.2016.04.005
5. Kojima, G. Frailty as a Predictor of Emergency Department Utilization Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J Am Med Directors Assoc (2019) 20(1):103–5. doi:10.1016/j.jamda.2018.10.004
6. Kojima, G. Frailty as a Predictor of Disabilities Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Disabil Rehabil (2017) 39(19):1897–908. doi:10.1080/09638288.2016.1212282
7. Crocker, TF, Brown, L, Clegg, A, Farley, K, Franklin, M, Simpkins, S, et al. Quality of Life Is Substantially Worse for Community-Dwelling Older People Living With Frailty: Systematic Review and Meta-Analysis. Qual Life Res (2019) 28(8):2041–56. doi:10.1007/s11136-019-02149-1
8. Kojima, G, Taniguchi, Y, Iliffe, S, and Walters, K. Frailty as a Predictor of Alzheimer Disease, Vascular Dementia, and All Dementia Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. J Am Med Directors Assoc (2016) 17(10):881–8. doi:10.1016/j.jamda.2016.05.013
9. Kojima, G, Iliffe, S, and Walters, K. Frailty Index as a Predictor of Mortality: A Systematic Review and Meta-Analysis. Age and ageing (2018) 47(2):193–200. doi:10.1093/ageing/afx162
10. Gray, WK, Richardson, J, McGuire, J, Dewhurst, F, Elder, V, Weeks, J, et al. Frailty Screening in Low-and Middle-Income Countries: A Systematic Review. J Am Geriatr Soc (2016) 64(4):806–23. doi:10.1111/jgs.14069
11. Payne, CF, Wade, A, Kabudula, CW, Davies, JI, Chang, AY, Gomez-Olive, FX, et al. Prevalence and Correlates of Frailty in an Older Rural African Population: Findings From the HAALSI Cohort Study. BMC Geriatr (2017) 17(1):293–10. doi:10.1186/s12877-017-0694-y
12. Barker, FJ, Davies, JI, Gomez-Olive, FX, Kahn, K, Matthews, FE, Payne, CF, et al. Developing and Evaluating a Frailty Index for Older South Africans—Findings From the HAALSI Study. Age and ageing (2021) 50(6):2167–73. doi:10.1093/ageing/afab111
13. Biritwum, R, Minicuci, N, Yawson, A, Theou, O, Mensah, G, Naidoo, N, et al. Prevalence of and Factors Associated With Frailty and Disability in Older Adults From China, Ghana, India, Mexico, Russia and South Africa. Maturitas (2016) 91:8–18. doi:10.1016/j.maturitas.2016.05.012
14. Hoogendijk, EO, Rijnhart, JJ, Kowal, P, Pérez-Zepeda, MU, Cesari, M, Abizanda, P, et al. Socioeconomic Inequalities in Frailty Among Older Adults in Six Low-And Middle-Income Countries: Results From the WHO Study on Global AGEing and Adult Health (SAGE). Maturitas (2018) 115:56–63. doi:10.1016/j.maturitas.2018.06.011
15. O’Caoimh, R, Galluzzo, L, Rodríguez-Laso, Á, Van der Heyden, J, Ranhoff, AH, Lamprini-Koula, M, et al. Prevalence of Frailty at Population Level in European ADVANTAGE Joint Action Member States: A Systematic Review and Meta-Analysis. Annali dell'Istituto superiore di sanita (2018) 54(3):226–38. doi:10.4415/ANN_18_03_10
16. Da Mata, FAF, Pereira, PPS, Andrade, KRC, Figueiredo, ACMG, Silva, MT, and Pereira, MG. Prevalence of Frailty in Latin America and the Caribbean: A Systematic Review and Meta-Analysis. PloS one (2016) 11(8):e0160019. doi:10.1371/journal.pone.0160019
17. Levett, TJ, Cresswell, FV, Malik, MA, Fisher, M, and Wright, J. Systematic Review of Prevalence and Predictors of Frailty in Individuals With Human Immunodeficiency Virus. J Am Geriatr Soc (2016) 64(5):1006–14. doi:10.1111/jgs.14101
18. Lewis, EG, Coles, S, Howorth, K, Kissima, J, Gray, W, Urasa, S, et al. The Prevalence and Characteristics of Frailty by Frailty Phenotype in Rural Tanzania. BMC Geriatr (2018) 18(1):283–11. doi:10.1186/s12877-018-0967-0
19. Lewis, EG, Wood, G, Howorth, K, Shah, B, Mulligan, L, Kissima, J, et al. Prevalence of Frailty in Older Community-Dwelling Tanzanians According to Comprehensive Geriatric Assessment. J Am Geriatr Soc (2018) 66(8):1484–90. doi:10.1111/jgs.15433
20. Gray, WK, Orega, G, Kisoli, A, Rogathi, J, Paddick, S-M, Longdon, AR, et al. Identifying Frailty and Its Outcomes in Older People in Rural Tanzania. Exp Aging Res (2017) 43(3):257–73. doi:10.1080/0361073X.2017.1298957
21. Adebusoye, LA, Cadmus, EO, Owolabi, MO, and Ogunniyi, A. Frailty and Mortality Among Older Patients in a Tertiary Hospital in Nigeria. Ghana Med J (2019) 53(3):210–6. doi:10.4314/gmj.v53i3.5
22. Witham, MD, Davies, JI, Bärnighausen, T, Bountogo, M, Manne-Goehler, J, Payne, CF, et al. Frailty and Physical Performance in the Context of Extreme Poverty: A Population-Based Study of Older Adults in Rural Burkina Faso. Wellcome open Res (2019) 4:135. doi:10.12688/wellcomeopenres.15455.1
23. Mwangala, PN, Mabrouk, A, Wagner, R, Newton, CR, and Abubakar, AA. Mental Health and Well-Being of Older Adults Living With HIV in Sub-Saharan Africa: A Systematic Review. BMJ open (2021) 11(9):e052810. doi:10.1136/bmjopen-2021-052810
24. Feng, Z, Lugtenberg, M, Franse, C, Fang, X, Hu, S, Jin, C, et al. Risk Factors and Protective Factors Associated With Incident or Increase of Frailty Among Community-Dwelling Older Adults: A Systematic Review of Longitudinal Studies. PloS one (2017) 12(6):e0178383. doi:10.1371/journal.pone.0178383
25. Verlaan, S, Ligthart-Melis, GC, Wijers, SL, Cederholm, T, Maier, AB, and de van der Schueren, MA. High Prevalence of Physical Frailty Among Community-Dwelling Malnourished Older Adults–A Systematic Review and Meta-Analysis. J Am Med Directors Assoc (2017) 18(5):374–82. doi:10.1016/j.jamda.2016.12.074
26. Mello, AC, Engstrom, EM, and Alves, LC. Health-Related and Socio-Demographic Factors Associated With Frailty in the Elderly: A Systematic Literature Review. Cadernos de saude publica (2014) 30:1143–68. doi:10.1590/0102-311x00148213
27. Kojima, G, Iliffe, S, and Walters, K. Smoking as a Predictor of Frailty: A Systematic Review. BMC Geriatr (2015) 15(1):131–7. doi:10.1186/s12877-015-0134-9
28. Tian, R, Almeida, OP, Jayakody, DM, and Ford, AH. Association Between Hearing Loss and Frailty: A Systematic Review and Meta-Analysis. BMC Geriatr (2021) 21(1):333–13. doi:10.1186/s12877-021-02274-y
29. Tan, BKJ, Man, REK, Gan, ATL, Fenwick, EK, Varadaraj, V, Swenor, BK, et al. Is Sensory Loss an Understudied Risk Factor for Frailty? A Systematic Review and Meta-Analysis. The Journals Gerontol Ser A (2020) 75(12):2461–70. doi:10.1093/gerona/glaa171
30. Saraiva, MD, Suzuki, GS, Lin, SM, de Andrade, DC, Jacob-Filho, W, and Suemoto, CK. Persistent Pain Is a Risk Factor for Frailty: A Systematic Review and Meta-Analysis From Prospective Longitudinal Studies. Age and ageing (2018) 47(6):785–93. doi:10.1093/ageing/afy104
31. Kojima, G, Taniguchi, Y, Kitamura, A, and Fujiwara, Y. Is Living Alone a Risk Factor of Frailty? A Systematic Review and Meta-Analysis. Ageing Res Rev (2020) 59:101048. doi:10.1016/j.arr.2020.101048
32. Kehler, DS, Hay, JL, Stammers, AN, Hamm, NC, Kimber, DE, Schultz, AS, et al. A Systematic Review of the Association Between Sedentary Behaviors With Frailty. Exp Gerontol (2018) 114:1–12. doi:10.1016/j.exger.2018.10.010
33. Pourmotabbed, A, Boozari, B, Babaei, A, Asbaghi, O, Campbell, MS, Mohammadi, H, et al. Sleep and Frailty Risk: A Systematic Review and Meta-Analysis. Sleep and Breathing (2020) 24(3):1187–97. doi:10.1007/s11325-020-02061-w
34. Kenya National Bureau of Statistics. Kenya Population and Housing Census Volume III. Nairobi: Distribution of Population by Age and Sex (2019).
35. Ministry of Health. Kenya STEPwise Survey for Non-Communicable Diseases Risk Factors 2015 Report. Kenya Nairobi: Ministry of Health (2015).
36. National AIDS and STI Control Programme. Preliminary KENPHIA 2018 Report. Nairobi: NASCOP (2020).
37. Kenya National Bureau of Statistics. The 2019 Kenya Population and Housing Census. Volume I (2019). Population by County and Sub-County 2019. Available From: http://housingfinanceafrica.org/app/uploads/VOLUME-I-KPHC-2019.pdf (Accessed March 9, 2022).
38. National AIDS Control Council (NACC). Kenya HIV County Profiles 2016. Kenya: National AIDS Control Council (2016).
39. Mombasa County Government. First County Integrated Development Plan 2013 (2013). Available From: http://kenyachamber.co.ke/wp-content/uploads/2017/02/1st-CIDP-2013-2017-Mombasa-County.pdf (Accessed April 13, 2022).
40. Kooij, KW, Wit, FW, Schouten, J, van der Valk, M, Godfried, MH, Stolte, IG, et al. HIV Infection Is Independently Associated With Frailty in Middle-Aged HIV Type 1-Infected Individuals Compared With Similar But Uninfected Controls. Aids (2016) 30(2):241–50. doi:10.1097/QAD.0000000000000910
41. Harris, PA, Taylor, R, Thielke, R, Payne, J, Gonzalez, N, and Conde, JG. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J Biomed Inform (2009) 42(2):377–81. doi:10.1016/j.jbi.2008.08.010
42. Abubakar, A, and Van De Vijver, FJ. How to Adapt Tests for Sub-Saharan Africa. In: Handbook of Applied Developmental Science in Sub-Saharan Africa. New York, NY: Springer (2017). p. 197–212.
43. Reinius, M, Wettergren, L, Wiklander, M, Svedhem, V, Ekström, AM, and Eriksson, LE. Development of a 12-Item Short Version of the HIV Stigma Scale. Health Qual Life Outcomes (2017) 15(1):115–9. doi:10.1186/s12955-017-0691-z
44. Üstün, TB, Chatterji, S, Kostanjsek, N, Rehm, J, Kennedy, C, Epping-Jordan, J, et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ (2010) 88:815–23. doi:10.2471/BLT.09.067231
45. Hays, RD, and DiMatteo, MR. A Short-Form Measure of Loneliness. J Personal Assess (1987) 51(1):69–81. doi:10.1207/s15327752jpa5101_6
46. Palmore, E. The Ageism Survey: First Findings. The gerontologist (2001) 41(5):572–5. doi:10.1093/geront/41.5.572
47. Rose, M, Yang, A, Welz, M, Masik, A, and Staples, M. Novel Modification of the Reported Edmonton Frail Scale. Australas J Ageing (2018) 37(4):305–8. doi:10.1111/ajag.12533
48. Richards, SJ, D’Souza, J, Pascoe, R, Falloon, M, and Frizelle, FA. Prevalence of Frailty in a Tertiary Hospital: A Point Prevalence Observational Study. Plos one (2019) 14(7):e0219083. doi:10.1371/journal.pone.0219083
49. Rubtsova, AA, Sabbag, S, Sundermann, E, Nguyen, AL, Ellis, RJ, Moore, DJ, et al. Frailty and Neurocognitive Impairment: Impacts on Quality of Life in HIV. The J Assoc Nurses AIDS Care JANAC (2020) 31(3):290–300. doi:10.1097/JNC.0000000000000142
50. Bristow, C, George, G, Hillsmith, G, Rainey, E, Urasa, S, Koipapi, S, et al. Low Levels of Frailty in HIV-Positive Older Adults on Antiretroviral Therapy in Northern Tanzania. J Neurovirol (2021) 27(1):58–69. doi:10.1007/s13365-020-00915-3
51. Edwards, A, Siedner, MJ, Nash, S, Neuman, M, Danaviah, S, Smit, T, et al. HIV Serostatus, Inflammatory Biomarkers and the Frailty Phenotype Among Older People in Rural KwaZulu-Natal, South Africa. Afr J AIDS Res (2020) 19(3):177–85. doi:10.2989/16085906.2020.1790398
52. Willig, AL, Overton, ET, and Saag, MS. The Silent Epidemic–Frailty and Aging With HIV. Total patient care in HIV & HCV (2016) 1(1):6–17.
53. Piovezan, RD, Poyares, D, and Tufik, S. Frailty and Sleep Disturbances in the Elderly: Possible Connections and Clinical Implications. Sleep Sci (2013) 6(4):175–9.
54. Lamont, RA, Swift, HJ, and Abrams, D. A Review and Meta-Analysis of Age-Based Stereotype Threat: Negative Stereotypes, Not Facts, Do the Damage. Psychol Aging (2015) 30(1):180–93. doi:10.1037/a0038586
55. Zora, S, Cella, A, Poli, S, Veronese, N, Zini, E, Giannoni, P, et al. Ageism" Is Associated With Self-Reported Multidimensional Frailty in Community-Dwelling Older Subjects: A Population-Based Study. Front Med (2021) 8:734636. doi:10.3389/fmed.2021.734636
56. Hawkins, KL, Zhang, L, Ng, DK, Althoff, KN, Palella, FJ, Kingsley, LA, et al. Abdominal Obesity, Sarcopenia, and Osteoporosis Are Associated With Frailty in Men Living With and Without HIV. AIDS (London, England) (2018) 32(10):1257–66. doi:10.1097/QAD.0000000000001829
57. Shah, K, Hilton, TN, Myers, L, Pinto, JF, Luque, AE, and Hall, WJ. A New Frailty Syndrome: Central Obesity and Frailty in Older Adults With the Human Immunodeficiency Virus. J Am Geriatr Soc (2012) 60(3):545–9. doi:10.1111/j.1532-5415.2011.03819.x
58. Crum-Cianflone, N, Roediger, MP, Eberly, L, Headd, M, Marconi, V, Ganesan, A, et al. Increasing Rates of Obesity Among HIV-Infected Persons During the HIV Epidemic. Plos one (2010) 5(4):e10106. doi:10.1371/journal.pone.0010106
59. Calmy, A, Hirschel, B, Cooper, DA, and Carr, A. A New Era of Antiretroviral Drug Toxicity. Antivir Ther (2009) 14(2):165–79. doi:10.1177/135965350901400203
60. Mwangala, PN, Wagner, RG, Newton, CR, and Abubakar, A. Navigating Life With HIV as an Older Adult on the Kenyan Coast: Perceived Health Challenges Seen Through the Biopsychosocial Model. Int J Public Health (2023) 68:1605916. doi:10.3389/ijph.2023.1605916
61. Melo, GC, Carvalho, ACA, Mendes, MLT, do Nascimento, RO, de Araújo, KCGM, Tanajura, DM, et al. Association Between Frailty Phenotype, Quantification of Plasma HIV-1 RNA, CD4 Cell Count and HAART in HIV-Positive Subjects: A Systematic Review and Meta-Analysis of Observational Studies. AIDS care (2021) 34:1–10. doi:10.1080/09540121.2021.1956414
62. Andrew, MK. Social Vulnerability in Old Age. In: Brocklehurst's Textbook of Geriatric Medicine and Gerontology. Elsevier (2010). p. 198–204.
63. Erlandson, KM, and Piggott, DA. Frailty and HIV: Moving From Characterization to Intervention. Curr HIV/AIDS Rep (2021) 18:157–75. doi:10.1007/s11904-021-00554-1
64. Han, CY, Miller, M, Yaxley, A, Baldwin, C, Woodman, R, and Sharma, Y. Effectiveness of Combined Exercise and Nutrition Interventions in Prefrail or Frail Older Hospitalised Patients: A Systematic Review and Meta-Analysis. BMJ open (2020) 10(12):e040146. doi:10.1136/bmjopen-2020-040146
65. De Labra Pinedo, C, Guimaraes-Pinheiro, C, Maseda, A, Lorenzo-López, T, and Millán-Calenti, JC. Effects of Physical Exercise Interventions in Frail Older Adults: A Systematic Review of Randomized Controlled Trials. BMC Geriatr (2015) 15. doi:10.1186/s12877-015-0155-4
66. Paw, MJ, van Uffelen, JG, Riphagen, I, and Wv, M. The Functional Effects of Physical Exercise Training in Frail Older People. Sports Med (2008) 38(9):781–93. doi:10.2165/00007256-200838090-00006
67. García-Esquinas, E, Graciani, A, Guallar-Castillón, P, López-García, E, Rodríguez-Mañas, L, and Rodríguez-Artalejo, F. Diabetes and Risk of Frailty and its Potential Mechanisms: A Prospective Cohort Study of Older Adults. J Am Med Directors Assoc (2015) 16(9):748–54. doi:10.1016/j.jamda.2015.04.008
68. Tuttle, LJ, Bittel, DC, Bittel, AJ, and Sinacore, DR. Early-Onset Physical Frailty in Adults With Diabesity and Peripheral Neuropathy. Can J Diabetes (2018) 42(5):478–83. doi:10.1016/j.jcjd.2017.12.001
69. Park, C, and Ko, FC. The Science of Frailty: Sex Differences. Clin Geriatr Med (2021) 37(4):625–38. doi:10.1016/j.cger.2021.05.008
Keywords: HIV, aging, frailty, correlates, sub-Saharan Africa
Citation: Mwangala PN, Nasambu C, Wagner RG, Newton CR and Abubakar A (2024) Prevalence and Factors Associated With Frailty Among Older Adults Living With HIV Compared to Their Uninfected Peers From the Kenyan Coast. Int J Public Health 69:1606284. doi: 10.3389/ijph.2024.1606284
Received: 06 June 2023; Accepted: 31 January 2024;
Published: 15 February 2024.
Edited by:
Olaf von dem Knesebeck, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Michel Oris, University of Geneva, SwitzerlandOne reviewer who chose to remain anonymous
Copyright © 2024 Mwangala, Nasambu, Wagner, Newton and Abubakar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick N. Mwangala, cGF0cmljay5ueml2b0Bha3UuZWR1, cG13YW5nYWxhMjdAZ21haWwuY29t
This Original Article is part of the IJPH Special Issue “Ageing and Health in Sub-Sahara Africa”
 Patrick N. Mwangala
Patrick N. Mwangala Carophine Nasambu2
Carophine Nasambu2