Abstract
Objectives:
This review aimed to assess the safety and efficacy of SARS-CoV-2 vaccines in patients with chronic liver disease (CLD).
Methods:
Cochrane Central Register of Controlled Trials, PubMed, Embase, and Web of Science were searched from 2020 to 2024. Data was extracted following Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines. The random-effects model (when I2 ≥ 50%) or fixed effect model (I2 < 50%) was used.
Results:
29 studies were included in this review. Compared to healthy controls (HCs), patients with CLD had a higher incidence of mild adverse events (RR = 1.60, p < 0.001), while the incidence of severe adverse events was similar (RR = 1.08, p = 0.92). Seropositivity rates of three antibodies in patients were lower than in HCs [neutralizing antibody (RR = 0.86, p = 0.002), anti-spike antibody (RR = 0.97, p = 0.06) and anti-receptor binding domain antibody (RR = 0.95, p = 0.04)]. Compared to unvaccinated patients, vaccinated patients had lower rates of SARS-CoV-2 infection, hospitalization and death (p ≤ 0.05).
Conclusion:
SARS-CoV-2 vaccines showed good safety and efficacy in CLD patients, but antibody response appeared to be decreased. Therefore, SARS-CoV-2 vaccines and booster doses should be given priority in this vulnerable population.
Introduction
The rapid development and deployment of vaccinations against SARS-CoV-2, alongside a degree of naturally acquired immunity from past infection, has transformed the landscape of the COVID-19 pandemic. At a population level, vaccination has been shown to reduce SARS-CoV-2 infection and protect against hospitalisation and death from severe COVID-19. However, understanding the immunogenicity and effectiveness of vaccination programmes in vulnerable cohorts with chronic disease remains an important clinical priority [1]. Patients with liver diseases might have worse outcome from COVID-19 than the general population [2–4]. Fortunately, vaccination is effective in preventing SARS-CoV-2 infection, severe symptom and death [5–7]. And societies in Europe, United States and China have recommended SARS-CoV-2 vaccination of all patients with CLD [8–10]. However, previous large cohort clinical trials of SARS-CoV-2 vaccines only included a few patients with CLD [11–13], and did not show the separate results of these patients. To our knowledge, studies on the safety and efficacy of SARS-CoV-2 vaccines in patients with CLD were still limited, and varied in populations, vaccine types and results. So, there is a need to further explore the safety and efficacy of SARS-CoV-2 vaccines in patients with CLD.
This systematic review and meta-analysis was performed to better understand the safety and efficacy of SARS-CoV-2 vaccines in patients with CLD, and it may be helpful for clinical practice.
Methods
Protocol and Registration
This systematic review and meta-analysis was conducted following a pre-established protocol according to the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines [14]. The protocol was initially registered in PROSPERO (registration number CRD42022302993) on 12 January 2022 [15].
Eligibility Criteria
Studies were eligible for being included in this systematic review and meta-analysis if they met the following criteria: 1) study included at least 20 adults aged ≥18 years with chronic liver disease of any severity or etiology (liver transplantation recipients were excluded) with/without COVID-19; 2) intervention was full-course vaccination (one dose: Johnson & Johnson, Cansino; two doses: other type of SARS-CoV-2 vaccines) of any type of SARS-CoV-2 vaccine with specific interval time; 3) intervention was compared with placebo, other vaccines or no vaccination; 4) outcomes included incidence of mild adverse events (MAEs), or incidence of severe adverse events (SAEs), or seropositivity/seroconversion rates of antibodies against SARS-CoV-2, or SARS-CoV-2 infection, or COVID-19-related hospitalization, or COVID-19-related mortality; 5) study type was randomized or non-randomized controlled trial, or cohort study, or case-control study, or cross-sectional study.
We included studies published in any kind of language. Review articles, case reports, animal studies, editorials, clinical guidelines, comment, meeting abstract, studies on CLD patients but only including liver transplant recipients (response to the vaccination and clinical outcomes are likely to be strongly influenced by the immunosuppressive medication rather than the status of liver disease), studies without separate outcomes of patients with chronic liver diseases, and studies retracted from publication were excluded.
Study Identification
We searched the Cochrane Central Register of Controlled Trials, PubMed, Embase, and Web of Science (from 2020 to 1 June 2024) for relevant articles. The Medical Subject Headings (MeSH) terms and free-text terms used were as follows: liver diseases, hepatic diseases, chronic liver diseases, cirrhosis, hepatitis, NAFLD, alcoholic liver disease, COVID-19, COVID-19 vaccines, SARS-CoV-2, vaccine, vaccination, immunization. Combination of these MeSH terms and free-text terms were used in each database. Relevant reviews and the reference list of the included articles were also checked to search for additional studies. The detailed searching strategies are shown in Supplementary Table S1.
Study Selection
Titles and abstracts of all articles were screened by two independent reviewers to assess whether they met inclusion criteria. Studies deemed eligible were then included in the full-text review by two independent reviewers. Disagreements were resolved by discussion or consulting a third reviewer, and the reasons for exclusion were recorded.
Data Extraction and Quality Assessment
The data were extracted by two independent reviewers and saved in a standardized form. Data extracted include the follows: participants (the number of participants, demographic and clinical characteristics), interventions and comparators (vaccine type, dose, comparator type, number of participants in intervention and comparison group, follow-up time after full-course vaccination), outcomes (the outcomes mentioned above, the unit of outcome), study designs (study type, location, date), study quality and study bias, other information: authors, publication time, etc.
The Newcastle-Ottawa Scale [16] was used to assess the quality of cohort study, and based on the total scores, cohort studies were classified as having low (7–9 stars), moderate (5–6 stars), and high (1–4 stars) risk of bias, respectively. The checklist recommended by Agency for Healthcare Research and Quality (AHRQ) [17] was used to assess the quality of cross-sectional study, and for each item of the checklist, 1 point (answered “yes”) or 0 point (answered “no” or “unclear”) was assigned. Based on the total scores, cross-sectional studies were classified as having low [8–11], moderate [4–7], and high (0–3) risk of bias, respectively. The assessment was completed by two reviewers independently, and the discrepancy was resolved through discussion or consulting a third reviewer.
Data Synthesis and Statistical Analysis
The safety outcomes were the incidence of MAEs, and incidence of SAEs. The efficacy outcomes were seropositivity/seroconversion rates of antibodies against SARS-CoV-2, SARS-CoV-2 infection, COVID-19-related hospitalization, and COVID-19-related mortality. A meta-analysis will be conducted when more than one study per outcome is identified. The Higgins statistic (I2) was used to assess the heterogeneity of data from different studies. The random-effects model will be used when I2 ≥ 50%, otherwise, the fixed effect model will be adopted. For dichotomous data (e.g., seropositivity rates), the levels were presented as rates (%) with 95% confidential interval (CI). Comparisons between rates were presented as risk ratio (RR) with 95% CI. All outcomes will be presented as forest plots, if appropriate. Subgroup analyses and meta-regression were not carried out due to the low number of studies. The funnel plots and Harbord’s test were used to evaluate the potential publication bias. A two-sided p-value less than 0.05 was considered significant. Review Manager 5.4.1 and Stata 12.0 were used for statistical analysis.
Results
Study Inclusion
6,893 records were identified through initial database searching, between which 2,269 records were removed records because of duplicates. Based on our inclusion and exclusion criteria, 4,536 records were excluded after title and abstract review, and further 29 records were excluded after full-text review. Ultimately, 29 studies were considered eligible and included in this literature review (Figure 1).
FIGURE 1
Flowchart summarizing the process for including the eligible studies. CLD, chronic liver disease (Global, 2022–2024).
Of the 29 included studies [6, 18–45] (Table 1–3), 19 were prospective cohort studies, 8 was retrospective study, and 2 was cross-sectional study. In the 29 included studies, all patients were older than 18 years, and 17 studies included CLD patients with cirrhosis. 22 studies had a control group (18 studies used healthy people as controls and 4 study used unvaccinated CLD patients as controls). 11 studies included inactivated vaccines, 3 inactivated and viral vector vaccines, 2 viral vector vaccines, 4 mRNA and viral vector vaccines, 8 mRNA SARS-CoV-2 vaccines, and 1 mRNA, inactivated and viral vector vaccines. The follow-up time after full-course vaccination of the most included studies were more than 7 days. Overall, 25 studies evaluated the safety and/or antibody response of SARS-CoV-2 vaccines [18–21, 24–38, 40–45] (Tables 1, 2), 4 study evaluated the clinical outcome (SARS-CoV-2 infection, hospitalization and death) after full-course SARS-CoV-2 vaccination [6, 22, 23, 39] (Table 3). Besides, the risk of publication bias of all included studies was low or moderate (Supplementary Tables S2–S4).
TABLE 1
| Study | Country | Study design | Patients with liver disease (% with cirrhosis) | Age (years) | Controls | Vaccine (number of vaccinated patients, %), dose | Follow-up time (days) after full-course vaccination | Incidence of mild adverse events | Incidence of severe adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Wang et al. [19] | China | Prospective cohort, multi-center study | 381 with NAFLD (0% with cirrhosis) | Median 39.0 (IQR 33.0–48.0) | — | Inactivated (BBIBP-CorV, 381, 100%), 2 doses | At least 14, median 39.0 (IQR, 35.0–50.0) | 29.4% within 28 days | 0% |
| Thuluvath, et al. [20] | United States | Prospective cohort study | 171 with CLD (46.2% with cirrhosis, 10 decompensated) | Mean 62.0 | — | mRNA (Moderna, 77, 45.0%; Pfizer, 80, 46.8%), 2 doses; Viral vector (Johnson & Johnson, 14, 8.2%), 1 dose | At least 28, mean 40.8 | Not available | 0% |
| Ruether et al. [21] | Germany | Prospective cohort study | 48 with CLD (100% with cirrhosis, 33.3% Child-Pugh class A, 37.5% Child-Pugh class B; 29.2% Child-Pugh class C) | Mean 53.8 (SD 9.5) | 52 healthy adults matched by age and vaccination regimen | mRNA (Pfizer, 38, 79.2%; Moderna, 6, 12.4%), 2 doses; Viral vector (AZD1222, 1, 2.1%), 2 doses; AZD1222+mRNA (3, 6.3%), 2 doses | At least 10, median 28 (IQR, 21–41) | Dose 1 39.6% in patients and 30.8% in controls; Dose 2 37.5% in patients and 30.8% in controls | Dose 1 2.1% in patients and 7.7% in controls; Dose 2 6.3% in patients and 5.8% in controls* |
| Xiang et al. [18] | China | Cross-sectional study | 149 with CHB (6.7% with compensated cirrhosis) | Median 41.0 (IQR 33.0–49.0) | — | Inactivated (BBIBP-CorV, CoronaVac, or WIBP-CorV, 149, 100%), 2 doses | At least 14, median 33 (IQR 24–48) | 30.2% within 7 days | 0% |
| Ai et al. [27] | China | Prospective cohort, multi-center study | 437 with CLD (35.0% with cirrhosis, 123 compensated cirrhosis, 30 decompensated) | Median 47.0 (IQR 38.0–56.0) | 144 healthy controls, age median 35.0 (IQR 28.5–41.5) | Inactivated (CoronaVac, BBIBP-CorV or WIBP-CorV, 437, 100%), 2 doses | At least 14 | 16% within 14 days of either dose | 0.20% |
| He et al. [24] | China | Cross-sectional study | 362 with CHB (13.3% with cirrhosis) | Median 45.0 (Range 19.0–78.0) | 87 healthy adults matched by age, gender and BMI | Inactivated (BBIBP-CorV/CoronaVac, 362, 100%), 2 doses | At least 21 (Range 21–105) | 14.1% in patients and 11.5% in controls within 30 days | 0% in patients, 0% in controls within 30 days |
| Calleri et al. [25] | Italy | Prospective cohort study | 89 with CLD (83.1% with cirrhosis) | Median 56.0 (IQR 50.0–62.0) | 30 healthy controls, median age 55.0 (IQR 46.0–59.0) | mRNA (Pfizer, 83, 93.3%; Moderna 6, 6.7%), 88.8% completed 2 doses | Median 23 (IQR 14–42) | Not available | 0% in patients, 0% in controls within 1 month |
| Bakasis et al. [26] | Greece | Prospective cohort study | 87 with CLD [43.7% with cirrhosis, MELD: median 9 (IQR 6–25)] | Median 67.0 (Range 27.0–86.0) | 40 healthy controls matched by age and gender | mRNA (Pfizer, 81, 93.1%; Moderna, 6, 6.9%), 2 doses | 1 month | Not available | 0% in patients, 0% in controls within 1 month |
| Biliotti et al. [44] | Italy | Prospective, single-center, observational study | 149 cirrhotic patients (100% with cirrhosis, 133 Child-Pugh A, 16 Child-Pugh B/C) | Median 60 (IQR 55–64) | 149 age and sex-matched HCWs | All cirrhotic patients: mRNA-1273 vaccine (Moderna); HCWs received the COVID-19 BNT162b2 vaccine (Pfizer-BioNTech) in 147 cases (98.7%) and the mRNA-1273 vaccine (Moderna) in 2 cases (1.3%) | 1 month | 101 (67.79) among patients with cirrhosis | 0% |
| Chen et al. [43] | China | Prospective observational study | 84 AILD (22.6% with cirrhosis) | Median 54.9 (IQR 49.3–60.8) | 68 healthcare workers | Inactivated (BBIBP-CorV or Corona-Vac, 84, 100%), 2 doses | 1 month (T1), 3 months (T2) and 6 months (T3) | 26.2% in patients with AILD within 7 days | 0% |
| Chen et al. [42] | China | Prospective observational study | 192 severe liver disease (66% with cirrhosis) | Median 53 (47–59) | 142 healthy controls and age median 48 (33–60) | Inactivated (BBIBP-CorV, 55, 29%; CoronaVac, 127, 66%; BBIBP-CorV + CoronaVac, 10, 5%), 2 doses | At least 21 days | 33.3% in patients and 12.0% in controls within 7 days | 0% |
| Li H. et al. [36] | China | Prospective observational study | 76 with autoimmune liver disease (26.3% with cirrhosis) | Median 54.0 (IQR 48.8–60.2) | 136 healthy controls age median 52.0 (33.0–62.2) | Inactivated (BBIBP-CorV: 21, 27.6%; CoronaVac: 49, 64.5%; BBIBP-CorV and CoronaVac: 6, 7.9%), 2, doses | At least 21 days | 25.0%% in patients and 17.6%% in controls within 7 days | 0% |
| Liu et al. [35] | China | Retrospective study | 210 cirrhotic patients (100% with cirrhosis) | Mean 46.95 (5.45) | 114 age‐matched vaccinated controls | Inactivated (CoronaVac, 210,100%), 2 doses | At least 14 days | 26.2%% in patients and 20.2%% in controls within 7 days | 0% |
| Ti et al. [32] | China | Retrospective and prospective epidemiological research | 153 patients with CHB (0% with cirrhosis) | 21∼68 (43.32 ± 12.65) | — | Inactivated vaccine, 153, 100%, 2 doses | At least 3 months | 18.30% patients with CHB | 0% |
| Wu et al. [29] | China | Prospective observational study | 200 CHB (6% with cirrhosis) | Mean 47.39 ± 13.60 | — | Inactivated (CoronaVac, 109, 2 doses); Viral vector (ZF2001, 91, 3 doses) | 2 weeks | 18.5% patients with CHB | 0% |
Characteristics of included studies on safety of SARS-CoV-2 vaccines (Global, 2022–2024).
*Incidence of severe adverse events of dose 2 was used for meta-analysis. BMI, body mass index; CHB, chronic hepatitis B; CLD, chronic liver disease; IQR, interquartile range; NAFLD, non-alcoholic fatty liver disease; SD, standard deviation.
TABLE 2
| Study | Country | Study design | Patients with liver disease (% with cirrhosis) | Age (years) | Controls | Vaccine (number of vaccinated patients, %), dose | Follow-up time (days) after full-course vaccination | Seropositivity rates |
|---|---|---|---|---|---|---|---|---|
| Wang, J. et al. 2021 [19] | China | Prospective cohort, multi-center study | 381 with NAFLD (0% with cirrhosis) | Median 39.0 (IQR 33.0–48.0) | — | Inactivated (BBIBP-CorV, 381, 100%), 2 doses | At least 14, median 39.0 (IQR, 35.0–50.0) | Neutralizing antibody, 95.5% |
| Thuluvath et al. [20] | United States | Prospective cohort study | 171 with CLD (46.2% with cirrhosis, 10 decompensated) | Mean 62.0 | — | mRNA (Moderna, 77, 45.0%; Pfizer, 80, 46.8%), 2 doses; Viral vector (Johnson & Johnson, 14, 8.2%), 1 dose | At least 28, mean 40.8 | Anti-SARS-CoV-2 spike antibody, 95.9% |
| Ruether et al. [21] | Germany | Prospective cohort study | 48 with CLD (100% with cirrhosis, 33.3% Child-Pugh class A, 37.5% Child-Pugh class B; 29.2% Child-Pugh class C) | Mean 53.8 (SD 9.5) | 52 healthy adults matched by age and vaccination regimen | mRNA (Pfizer, 38, 79.2%; Moderna, 6, 12.4%), 2 doses; Viral vector (AZD1222, 1, 2.1%), 2 doses; AZD1222+mRNA (3, 6.3%), 2 doses | At least 10, median 28 (IQR, 21–41) | Anti-spike antibody 98% in patients and 100% in healthy controls; Anti-S RBD antibody 94% in patients and 100% in healthy controls |
| Xiang et al. [18] | China | Cross-sectional study | 149 with CHB (6.7% with compensated cirrhosis) | Median 41.0 (IQR 33.0–49.0) | — | Inactivated (BBIBP-CorV, CoronaVac, or WIBP-CorV, 149, 100%), 2 doses | At least 14, median 33 (IQR 24–48) | Anti-S-RBD IgG, 87.25%; neutralizing antibody 74.5% |
| Ai et al. [27] | China | Prospective cohort, multi-center study | 437 with CLD (35.0% with cirrhosis, 123 compensated cirrhosis, 30 decompensated) | Median 47.0 (IQR 38.0–56.0) | 144 healthy controls, age median 35.0 (IQR 28.5–41.5) | Inactivated (CoronaVac, BBIBP-CorV or WIBP-CorV, 437, 100%), 2 doses | At least 14 | Neutralizing antibody 77.3% in patients and 90.3% in healthy controls |
| He et al. [24] | China | Cross-sectional study | 362 with CHB (13.3% with cirrhosis) | Median 45.0 (Range 19.0–78.0) | 87 healthy adults matched by age, gender and BMI. | Inactivated (BBIBP-CorV/CoronaVac, 362, 100%), 2 doses | At least 21 (Range 21–105) | Anti-spike IgG 97.8% in patients and 100.0% in controls; Anti-RBD IgG 98.3% in patients and 100% in controls; Neutralizing antibody 72.6% in patients and 77.4% in controls |
| Calleri et al. [25] | Italy | Prospective cohort study | 89 with CLD (83.1% with cirrhosis) | Median 56.0 (IQR 50.0–62.0) | 30 healthy controls, median age 55.0 (IQR 46.0–59.0) | mRNA (Pfizer, 83, 93.3%; Moderna 6, 6.7%), 88.8% completed 2 doses | Median 23 (IQR 14–42) | Anti-spike IgG 94.9% in patients and 100% in controls |
| Bakasis et al. 2022 [26] | Greece | Prospective cohort study | 87 with CLD [43.7% with cirrhosis, MELD: median 9 (IQR 6–25)] | Median 67.0 (Range 27.0–86.0) | 40 healthy controls matched by age and gender | mRNA (Pfizer, 81, 93.1%; Moderna, 6, 6.9%), 2 doses | 1 month | Anti-spike IgG 92.0% in patients and 100% in controls; Neutralizing antibody 89.7% in patients and 100% in controls |
| Al-Dury et al. [45] | Sweden | Prospective cohort study | 48 with cirrhosis (100% with cirrhosis, 31 Child-Pugh A; 15 Child-Pugh B; 2 Child-Pugh C) | Median 63.5 (26–76) | 39 healthy controls 60 (25–86) | mRNA (Moderna, 4, 8%; Pfizer-BioNTech, 44, 92%), 2 dose | 6 months | Anti-RBD IgG 98% in patients and 100% in controls |
| Biliotti et al. [44] | Italy | Prospective, single-center, observational study | 149 cirrhotic patients (100% with cirrhosis, 133 Child-Pugh A, 16 Child-Pugh B/C) | Median 60 (55–64) | 149 age and sex-matched healthcare workers | All cirrhotic patients: mRNA-1273 vaccine (Moderna); HCWs received the COVID-19 BNT162b2 vaccine (Pfizer-BioNTech) in 147 cases (98.7%) and the mRNA-1273 vaccine (Moderna) in 2 cases (1.3%) | 1 month | anti-S antibodies 100% in cirrhotic patients and HCWs |
| Chen et al. [43] | China | Prospective observational study | 84 AILD (22.6% with cirrhosis) | Median 54.9 (49.3–60.8) | 68 healthcare workers | Inactivated (BBIBP-CorV or Corona-Vac, 84, 100%), 2 doses | 1 month (T1), 3 months (T2) and 6 months (T3) | Anti-RBD IgG 90% in patients and 100% in controls; Neutralizing antibody 90% in patients and 100% in controls |
| Chen et al. [42] | China | Prospective observational study | 192 severe liver disease (66% with cirrhosis) | Median 53 (IQR 47–59) | 142 healthy controls and age median 48 (IQR 33–60) | Inactivated (BBIBP-CorV, 55, 29%; CoronaVac, 127, 66%; BBIBP-CorV + CoronaVac, 10, 5%),2 doses | At least 21 days | Anti-RBD IgG 98.4% in patients and 100% in controls; Neutralizing antibody 57.8% in patients and 76.1% in controls |
| Duengelhoef et al. [41] | Germany | Prospective observational cohort study | 112 consecutive patients with AIH (35% with cirrhosis) and 144 consecutive patients with cholestatic liver disease (17% with cirrhosis) | AIH 53 (17); PBC/PSC 52 (15) | 95 healthy controls age 51 (8) | mRNA (BNT162b2; BioNTech SE/Pfizer or mRNA‐1273; Moderna Biotech); Viral vector vaccine (AZD1222; AstraZeneca). 2 doses | >2 weeks | Anti-spike antibody 98.2% in patients and 100% in healthy controls; Anti-RBD IgG 99.5% in patients and 100% in controls |
| Goel et al. [40] | India | Prospective observational cohort study | 131 cirrhotic patients (61.1% with decompensated cirrhosis) | Median 50 (IQR 43–58) | — | Viral vector vaccine (AZD1222; AstraZeneca). 2 doses | 4 weeks | Anti-spike antibody 99.2%% in patients; Neutralizing antibody 84% in patients |
| Kulkarni et al. [38] | India | Single-center prospective study | 50 non-cirrhosis CLD and 113 Cirrhosis (69%) | NCCLD:49.34 ± 10.48; Cirrhosis: 52.42 ± 9.93 | 60 healthy controls age 51.2 ± 8.75 | Viral vector vaccine (Covishield, 124, 76.07%); inactivated vaccines (Covaxin, 39, 23.93%), 2 dose | 3 months | Anti-spike antibody 84.05% in patients and 91.7% in healthy controls |
| Li et al. [36] | China | Cross-sectional study with longitudinal follow-up | 137 patients with liver dysfunction (47.5% with cirrhosis) | Mean 50.2 | 134 healthy controls and age mean 42.6 | Inactivated:113, 82.5%, 2 doses; RBD-subunit recombinant: 24, 17.5%, 3 doses | At least 30 days | Neutralizing antibody 95.0%% in patients and 96.0% in healthy controls |
| Li et al. [36] | China | Prospective observational study | 76 with autoimmune liver disease (26.3% with cirrhosis) | Median 54.0 (IQR 48.8–60.2) | 136 healthy controls age median52.0 (IQR 33.0–62.2) | Inactivated (BBIBP-CorV: 21, 27.6%; CoronaVac: 49, 64.5%; BBIBP-CorV and CoronaVac: 6, 7.9%), 2, doses | At least 21 days | Anti-RBD IgG 97.4% in patients and 100% in controls; Neutralizing antibody 63.2% in patients and 84.6% in healthy controls |
| Liu et al. [34] | China | Prospective observational study | 237 CLD (22.36% with cirrhosis) | Mean 47.01 (12.00) | 170 healthy controls (HCs) of similar age and post-vaccination days | Inactivated (BBIBP-CorV; CoronaVac; WIBP-CorV) | At least 120 days | Anti-RBD IgG 87.34% in patients and 93.75% in controls; Neutralizing antibody 72.73%% in patients and 100%% in healthy controls |
| Singh et al. 2023 [33] | India | Retrospective study | 88 Cirrhosis (15 CTP A, 71 CTP B and 2 CTP C) | Mean 53.3 ± 10.08 | — | Viral vector (ChAdOx1-nCOV, 88, 100%), 2 doses | 39 (23–76) days | Anti-spike antibody 92.05% in patients |
| Ti et al. [32] | China | Retrospective and prospective epidemiological research | 153 patients with CHB (0% with cirrhosis) | 21∼68 (43.32 ± 12.65) | — | Inactivated vaccine, 153, 100%, 2 doses | At least 3 months | Neutralizing antibody 45.50% in patients |
| Willauer et al. [31] | United States | Retrospective study | 24 CLD (29% with cirrhosis) | Mean 61.0 ± 9.0 | 9 healthy controls and age 51.0 ± 14.5 | mRNA (Pfizer/BioNTech (BNT162b2), 13 54%; Moderna (mRNA-1273) 11 46%), 2 doses | 31 days (23–103) | Anti-spike antibody 95% in patients and 95.6% in healthy controls; Neutralizing antibody 95% in patients and 100% in healthy controls |
| Willuweit et al. [30] | Germany | Prospective observational study | 110 Cirrhosis (69% Child A, 28% Child B and 3% Child C) | Median 55(IQR 45–66) | 80 HCWs and age median 54 (IQR 45–59) | mRNA (BNT162b2 (Pfizer-BioNTech) 100%), 2 doses | 69 days (43–106) | Anti-spike antibody 96% in patients and 99% in healthy controls |
| Wu et al. [29] | China | Prospective observational study | 200 CHB (6% with cirrhosis) | Mean 47.39 ± 13.60 | — | Inactivated (CoronaVac,109,2 doses); Viral vector (ZF2001, 91, 3 doses) | 2 weeks | Neutralizing antibody 86.1%% in patients |
| Yang et al. 2023 [28] | China | Prospective multicenter study | 261 chronic liver disease (79 compensated advanced CLD and 33 decompensated advanced CLD) | Non-ACLD: 38.0 (34.0, 47.0); CACLD: 55.0 (48.0, 59.0); DACLD: 54.0 (48.0, 59.0) | 106 healthy controls and age median 46.0 (IQR 36.0, 54.8) | Inactivated (CoronaVac or BBIBP-CorV, 100%), 3, doses | 6 months | Neutralizing antibody 73.18% in patients and 79.2% in healthy controls; Anti-spike antibody 77.39% in patients and 82.1% in healthy controls |
Characteristics of included studies on antibody response of SARS-CoV-2 vaccines (Global, 2022–2024).
TABLE 3
| Study | Country | Study design | Patients with liver disease (% with cirrhosis) | Age (years) | Controls | Vaccine, dose (number of vaccinated patients, %) | Follow-up time (days) after full-course vaccination | SARS-CoV2 infection | Hospitalization for COVID-19 | COVID-19 related death |
|---|---|---|---|---|---|---|---|---|---|---|
| John et al. [6] | United States | Retrospective cohort study | 20,037 patients with cirrhosis (84.3% CTP A, 15.1% CTP B, 0.6% CTP C) | Median 69.1 (IQR 64.9–73.3) | 20037 matched unvaccinated cirrhotic patients | mRNA vaccines (Pfizer/Moderna, 100%), 62.7% completed 2 doses | At least 7 days | 0.03% in vaccinated patients and 0.14% in unvaccinated patients | 0% in vaccinated patients and 0.02% in unvaccinated patients | 0% in vaccinated patients and 0.01% in unvaccinated patients |
| John et al. [23] | United States | Retrospective cohort study | 254 COVID-19 patients with cirrhosis (76.8% CTP A, 21.7% CTP B, 1.6% CTP C) | Median 63.8 (IQR 58.6–69.0) | 508 matched unvaccinated COVID-19 patients with cirrhosis | mRNA vaccines (Pfizer, 126, 49.6%; Moderna, 121, 47.6%), 2 doses; Viral vector (Johnson & Johnson, 7, 2.8%), 1 dose. 32.3% completed full-course vaccination | At least 14 days | Not available | Not available | 3.7% in vaccinated patients and 14.6% in unvaccinated patients |
| Moon et al. [22] | United States | Retrospective cohort study | 21 COVID-19 patients with CLD (51% CTP A, 29% CTP B, 5% CTP C) | Median 59.0 (Range 28.0–72.0) | 225 unvaccinated COVID-19 patients with CLD, median age 59.0 | mRNA (Pfizer, 4, 19.0%; Moderna, 1, 4.8%), 2 doses; Inactivated (Bharat Biotech, 2, 9.5%; Sinovac, 1, 4.8%), 2 doses; Viral vector (Oxford-AZ, 12, 57.1%), 2 doses; Viral vector (Cansino, 1, 4.8%), 1 dose. 42.9% completed full-course vaccination | At least 14 days, median 21 days | Not available | 33.3% in vaccinated patients and 72.0% in unvaccinated patients | 0% in vaccinated patients and 8.0% in unvaccinated patients |
| Ivashkin et al. [39] | Russia | Retrospective cohort study | 89 patients with cirrhosis (58.4% CTP A and 41.6% CTP B/C) | Median 59 (IQR 48–68) | 148 matched unvaccinated cirrhotic patients | Viral vector: Gam-COVID-Vac (Sputnik V), 2 doses | At least 17 days | 4.49% in vaccinated patients and 16.21% in unvaccinated patients | 0% in vaccinated patients and 8.10% in unvaccinated patients | 0% in vaccinated patients and 6.76% in unvaccinated patients |
Characteristics of included studies on clinical outcomes of SARS-CoV-2 vaccines (Global, 2022–2024).
Safety of SARS-CoV-2 Vaccination
Among the 15 studies reporting the safety of the SARS-CoV-2 vaccines, 12 had available results of MAEs, 15 had available results of SAEs, 5 had available results of MAEs of healthy controls, and 7 had available results of SAEs of healthy controls [18–21, 24–27, 29, 32, 35, 36, 42–44] (Table 1). In all 15 studies (2788 CLD patients), most adverse events were mild, and only six patients had SAEs (including local pain/swelling, fever, fatigue, headache, muscle pain, joint pain, diarrhea, and grade 3 ALT elevation) after SARS-CoV-2 vaccination. The results of meta-analysis showed that incidence of MAEs was 28.0% (95% CI 21.0%–36.0%) in CLD patients (Supplementary Table S5; Supplementary Figure S1A), and incidence of SAEs was 1.0% (95% CI 0%–27.0%) in CLD patients (Supplementary Table S5; Supplementary Figure S1B). Compared to healthy controls, CLD patients had higher incidence of MAEs (RR 1.60, 95% CI 1.27–2.02, p < 0.001) (Supplementary Figure S2A), while had similar incidence of SAEs (RR 1.08, 95% CI 0.23–5.11, p = 0.92) (Supplementary Figure S2B).
Antibody Response of SARS-CoV-2 Vaccination
In the 25 studies on the antibody response to SARS-CoV-2 vaccines, 5 determined the neutralizing antibody, 4 determined anti-spike antibody and neutralizing antibody, 2 determined anti-spike antibody and anti-receptor binding domain (RBD) antibody, 1 determined anti-RBD IgG, 7 determined anti-spike antibody, 5 determined neutralizing antibody and anti-RBD IgG, and 1 determined neutralizing antibody, anti-spike antibody and anti-RBD antibody [18–21, 24–38, 40–44] (Table 2). 18 studies had healthy controls. The results of meta-analysis showed seropositivity rates of neutralizing antibody, anti-spike antibody and anti-RBD antibody were 79.0% (95% CI 72.0%–87.0%), 94.0% (95% CI 91.0%–97.0%) and 96.0% (95% CI 93.0%–98.0%) in CLD patients, respectively (Supplementary Table S5). Compared to healthy controls, CLD patients had lower seropositivity rates of neutralizing antibody (RR 0.86, 95% CI 0.79–0.95, p = 0.002) (Figure 2A), anti-spike antibody (RR 0.97, 95% CI 0.95–1.00, p = 0.06) (Figure 2B) and anti-RBD antibody (RR 0.95, 95% CI 0.90–1.00, p = 0.04) (Figure 2C). Due to the fact that in evaluating the response of anti-spike antibody and anti-RBD antibody in patients with chronic liver disease after vaccination, some of the subjects in the literature were all patients with cirrhosis, we further conducted subgroup analysis, and the results remained unchanged (Supplementary Figures S3, S4).
FIGURE 2
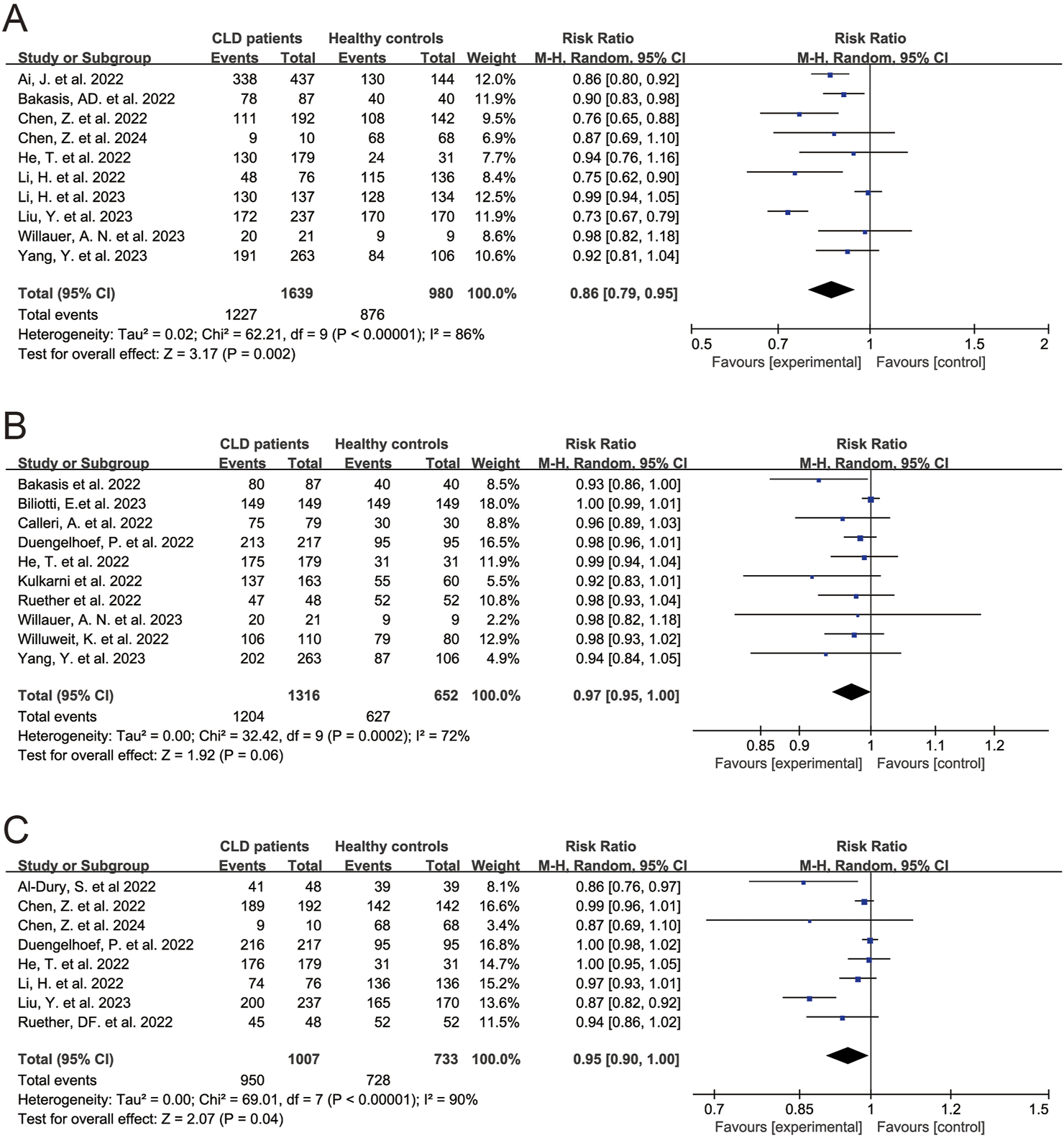
Forest plots of the comparison of the seropositivity rates of SARS-CoV-2 antibody between chronic liver disease patients and healthy controls. (A) Neutralizing antibody. (B) Anti-spike antibody. (C) Anti-receptor binding domain antibody. p < 0.05 was considered significant. CI, confidential interval; CLD, chronic liver disease; RBD, receptor binding domain (Global, 2022–2024).
Clinical Outcome After SARS-CoV-2 Vaccination
Four study assessed the association between SARS-CoV-2 vaccination and clinical outcome [6, 22, 23, 39] (Table 3). The results indicated that, compared to unvaccinated CLD patients, CLD patients after full-course vaccination of SARS-CoV-2 vaccines had lower rates of SARS-CoV-2 infection (RR 0.25, 95% CI 0.11–0.55, p < 0.001) (Figure 3A), COVID-19-related hospitalization (RR 0.28, 95% CI 0.12–0.66, p = 0.003) (Figure 3B) and death (RR 0.23, 95% CI 0.09–0.58, p = 0.002) (Figure 3C).
FIGURE 3
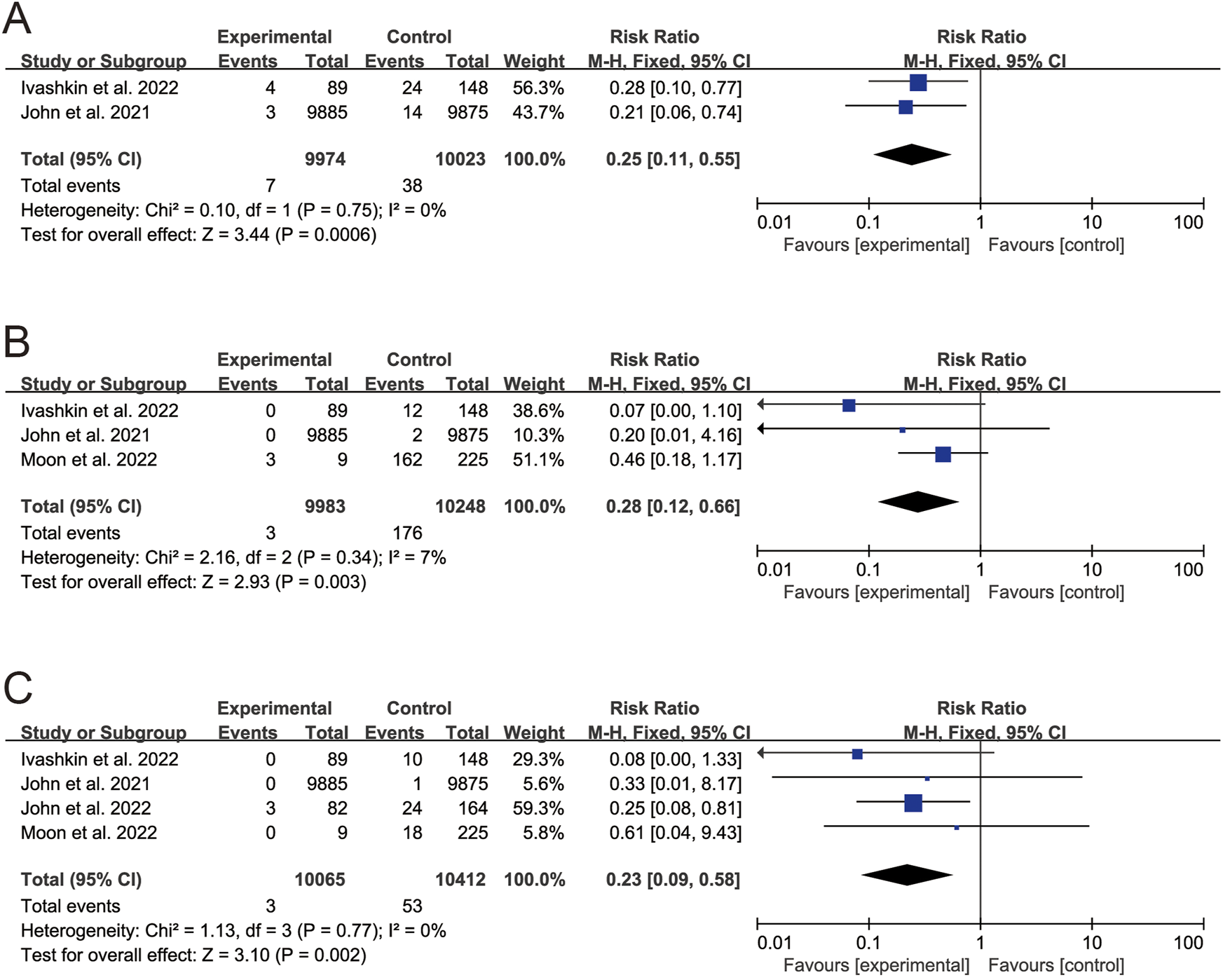
Forest plots of the comparison of the clinical outcome between vaccinated patients and unvaccinated patients. (A) SARS-CoV-2 infection. (B) COVID-19-related hospitalization. (C) COVID-19-related death. p < 0.05 was considered significant. CI, confidential interval; CLD, chronic liver disease (Global, 2022–2024).
Publication Bias
The funnel plots showed no obvious asymmetry (Supplementary Figure S5), which indicated there might be no publication bias. Due to small number of eligible studies, only three outcomes (seropositivity rates of anti-spike antibody, neutralizing antibody, and COVID-19-related death) could be used to perform the Harbord’s test, and the result also indicated no publication bias (all p > 0.05) (Supplementary Table S6).
Discussion
This systematic review and meta-analysis focused on the safety and efficacy of SARS-CoV-2 vaccines in patients with CLD. By analyzing the 29 eligible studies, SARS-CoV-2 vaccines were revealed to be safe in CLD patients. Full-course vaccination of SARS-CoV-2 vaccines induced promising antibody response (seropositivity rates of three antibodies were all higher than 80%) in CLD patients, but the seropositivity rates were lower in CLD patients than in healthy controls, which might decrease the immune protection provided by vaccination. Furthermore, full-course vaccination of SARS-CoV-2 vaccines may reduce the SARS-CoV-2 infection, COVID-19-related hospitalization and death in CLD patients.
The safety of SARS-CoV-2 vaccines is a highly concerned issue, and some previous studies reported thrombosis [46] and myocarditis cases [47] after SARS-CoV-2 vaccination. In this review, most AEs of CLD patients were mild, and the SAEs of CLD patients were rare. And the incidences of AEs were similar between CLD patients and HCs. Moreover, no thrombosis or myocarditis was reported. So, the results indicated good safety of SARS-CoV-2 vaccines in CLD patients.
CLD patients have dysregulated innate and adaptive immunity, which might weaken the immune response to vaccine [9]. In this review, the results of meta-analysis revealed that the seropositivity rates of SARS-CoV-2 antibody tended to be lower in CLD patients than in healthy controls, which indicated CLD might also weaken patients’ immune response to COVID-19 vaccine. Whereas, full-course vaccination of SARS-CoV-2 vaccines could still induce considerable antibody response in CLD patients (seropositivity rates of three antibodies were all higher than 80%). Furthermore, SARS-CoV-2 vaccination brought significant clinical benefit to CLD patients (vaccinated patients had significant lower proportion of SARS-CoV-2 infection, COVID-19-related hospitalization and death than that in unvaccinated patients). Therefore, SARS-CoV-2 vaccines had good efficacy in CLD patients.
Strengths of this study are as follows: first, this study was conducted following a pre-established protocol and guidelines, and different databases were used for including eligible studies, which helped to improve the quality of this study; Second, so far, there is no random controlled trial with large samples on CLD patients. In this context, this study is the first systematic review and meta-analysis on the safety and efficacy of SARS-CoV-2 vaccines in CLD patients, so it may provide relatively high-quality evidence for clinical practice. This study still has several limitations. First, due to lack of related data, this study did not assess the long-term efficacy of SARS-CoV-2 vaccines in CLD patients. Second, the sample of included studies were relatively small, and there was no random controlled trial (RCT) with large samples on CLD patients. Third, in the literature included in the meta-analysis, the subjects mainly had mild chronic liver disease, and no subgroup analysis was conducted for liver diseases of different severity levels. Forth, the recent emergence and global spreading of omicron subvariants have shown striking antibody evasion [48] and posed a critical challenge to the efficacy of SARS-CoV-2 vaccines. But until now, no study explored the efficacy of SARS-CoV-2 vaccines in CLD patients against omicron subvariants. So, there is a need for the studies on the long-term efficacy of SARS-CoV-2 vaccines and the efficacy of SARS-CoV-2 vaccines against omicron subvariants in CLD patients, and large-sample RCT.
In conclusion, SARS-CoV-2 vaccines showed good safety and efficacy in CLD patients. However, antibody response appeared to be lower in CLD patients than in healthy controls. Therefore, SARS-CoV-2 vaccines and booster doses should be given priority in this vulnerable population.
Statements
Author contributions
GX, TH, BZ, ZY, and HR contributed to the conception and design of the systematic review. GX, TH, BZ, and ZY filed the PROSPERO registration. GX and TH carried out the systematic search, study analysis and wrote the initial draft of the manuscript. HR, DC, MP, GZ, PH, DZ, MC, and NL contributed to the manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Remarkable Innovation-Clinical Research Project and the Joint Project of Pinnacle Disciplinary Group of The Second Affiliated Hospital of Chongqing Medical University, the first batch of key disciplines on public health in Chongqing, Health Commission of Chongqing, China.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2024.1605295/full#supplementary-material
Abbreviations
AHRQ, Agency for Healthcare Research and Quality; ALT, alanine aminotransferase; BMI, body mass index; CHB, chronic hepatitis B; CI, confidential interval; CLD, chronic liver disease; COVID-19, coronavirus disease 2019; IQR, interquartile range; MAEs, mild adverse events; MeSH, Medical Subject Headings; NAFLD, non-alcoholic fatty liver disease; OR, odds ratio; RBD, receptor binding domain; RCT, random controlled trial; RR, risk ratio; SAEs, serious adverse events; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; WHO, world health organization.
References
1.
MurraySMPoseEWittnerMLondoñoMCSchaubGCookJet alImmune Responses and Clinical Outcomes After COVID-19 Vaccination in Patients With Liver Disease and Liver Transplant Recipients. J Hepatol (2024) 80(1):109–23. 10.1016/j.jhep.2023.10.009
2.
MarjotTMoonAMCookJAAbd-ElsalamSAlomanCArmstrongMJet alOutcomes Following SARS-CoV-2 Infection in Patients With Chronic Liver Disease: An International Registry Study. J Hepatol (2021) 74(3):567–77. 10.1016/j.jhep.2020.09.024
3.
KovalicAJSatapathySKThuluvathPJ. Prevalence of Chronic Liver Disease in Patients With COVID-19 and Their Clinical Outcomes: A Systematic Review and Meta-Analysis. Hepatol Int (2020) 14(5):612–20. 10.1007/s12072-020-10078-2
4.
WuJYuJShiXLiWSongSZhaoLet alEpidemiological and Clinical Characteristics of 70 Cases of Coronavirus Disease and Concomitant Hepatitis B Virus Infection: A Multicentre Descriptive Study. J Viral Hepat (2021) 28(1):80–8. 10.1111/jvh.13404
5.
SadoffJGrayGVandeboschACardenasVShukarevGGrinsztejnBet alSafety and Efficacy of Single-Dose Ad26.COV2.S Vaccine Against COVID-19. N Engl J Med (2021) 384(23):2187–201. 10.1056/NEJMoa2101544
6.
JohnBVDengYScheinbergAMahmudNTaddeiTHKaplanDet alAssociation of BNT162b2 mRNA and mRNA-1273 Vaccines With COVID-19 Infection and Hospitalization Among Patients With Cirrhosis. JAMA Intern Med (2021) 181(10):1306–14. 10.1001/jamainternmed.2021.4325
7.
ThompsonMGBurgessJLNalewayALTynerHYoonSKMeeceJet alPrevention and Attenuation of Covid-19 With the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med (2021) 385(4):320–9. 10.1056/NEJMoa2107058
8.
CornbergMButiMEberhardtCSGrossiPAShouvalD. EASL Position Paper on the Use of COVID-19 Vaccines in Patients With Chronic Liver Diseases, Hepatobiliary Cancer and Liver Transplant Recipients. J Hepatol (2021) 74(4):944–51. 10.1016/j.jhep.2021.01.032
9.
FixOKBlumbergEAChangKMChuJChungRTGoacherEKet alAmerican Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology (2021) 74(2):1049–64. 10.1002/hep.31751
10.
Chinese Society of Hepatology CMA. Rapid Guideline for COVID-19 Vaccination in Patients With Chronic Liver Diseases, Hepatolbiliary Malignancy and Liver Transplant. Zhonghua Gan Zang Bing Za Zhi (2021) 29(6):523–6. 10.3760/cma.j.cn501113-20210612-00278
11.
PolackFPThomasSJKitchinNAbsalonJGurtmanALockhartSet alSafety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med (2020) 383(27):2603–15. 10.1056/NEJMoa2034577
12.
BadenLREl SahlyHMEssinkBKotloffKFreySNovakRet alEfficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med (2021) 384(5):403–16. 10.1056/nejmoa2035389
13.
VoyseyMClemensSACMadhiSAWeckxLYFolegattiPMAleyPKet alSafety and Efficacy of the ChAdOx1 nCoV-19 Vaccine (AZD1222) Against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet (2021) 397(10269):99–111. 10.1016/S0140-6736(20)32661-1
14.
MoherDLiberatiATetzlaffJAltmanDGGroupP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med (2009) 151(4):264–W64. W64. 10.7326/0003-4819-151-4-200908180-00135
15.
TaiyuHBiqiongZZiqiaoYNingLMingliPDachuanCet alSafety and Efficacy of COVID-19 Vaccines in Patients With Chronic Liver Diseases: A Systematic Review and Meta-Analysis. PROSPERO 2022 CRD42022302993. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=302993 (Accessed November 15, 2024).
16.
WellsGASheaBO'ConnellDPetersonJWelchVLososMet alThe Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed November 15, 2024).
17.
RostomADubeCCranneyASaloojeeNSyRGarrittyCet alCeliac Disease. Evid Rep Technol Assess (Summ) (2004) 104:1–6.
18.
XiangTLiangBWangHQuanXHeSZhouHet alSafety and Immunogenicity of a SARS-CoV-2 Inactivated Vaccine in Patients With Chronic Hepatitis B Virus Infection. Cell Mol Immunol (2021) 18(12):2679–81. 10.1038/s41423-021-00795-5
19.
WangJHouZLiuJGuYWuYChenZet alSafety and Immunogenicity of COVID-19 Vaccination in Patients With Non-Alcoholic Fatty Liver Disease (CHESS2101): A Multicenter Study. J Hepatol (2021) 75(2):439–41. 10.1016/j.jhep.2021.04.026
20.
ThuluvathPJRobartsPChauhanM. Analysis of Antibody Responses After COVID-19 Vaccination in Liver Transplant Recipients and Those With Chronic Liver Diseases. J Hepatol (2021) 75(6):1434–9. 10.1016/j.jhep.2021.08.008
21.
RuetherDFSchaubGMDuengelhoefPMHaagFBrehmTTFathiAet alSARS-CoV2-Specific Humoral and T-Cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin Gastroenterol Hepatol (2022) 20(1):162–72.e9. 10.1016/j.cgh.2021.09.003
22.
MoonAMWebbGJGarcía-JuárezIKulkarniAVAdaliGWongDKet alSARS-CoV-2 Infections Among Patients With Liver Disease and Liver Transplantation Who Received COVID-19 Vaccination. Hepatol Commun (2022) 6(4):889–97. 10.1002/hep4.1853
23.
JohnBVDengYSchwartzKBTaddeiTHKaplanDEMartinPet alPostvaccination COVID-19 Infection Is Associated With Reduced Mortality in Patients With Cirrhosis. Hepatology (2022) 76(1):126–38. 10.1002/hep.32337
24.
HeTZhouYXuPLingNChenMHuangTet alSafety and Antibody Response to Inactivated COVID-19 Vaccine in Patients With Chronic Hepatitis B Virus Infection. Liver Int (2022) 42(6):1287–96. 10.1111/liv.15173
25.
CalleriASaraccoMPittalugaFCavalloRRomagnoliRMartiniS. Seroconversion After Coronavirus Disease 2019 Vaccination in Patients Awaiting Liver Transplantation: Fact or Fancy?Liver Transpl (2022) 28(2):180–7. 10.1002/lt.26312
26.
BakasisADBitzogliKMouziourasDPouliakisARoumpoutsouMGoulesAVet alAntibody Responses After SARS-CoV-2 Vaccination in Patients With Liver Diseases. Viruses (2022) 14(2):207. 10.3390/v14020207
27.
AiJWangJLiuDXiangHGuoYLvJet alSafety and Immunogenicity of SARS-CoV-2 Vaccines in Patients With Chronic Liver Diseases (CHESS-NMCID 2101): A Multicenter Study. Clin Gastroenterol Hepatol (2022) 20(7):1516–24.e2. 10.1016/j.cgh.2021.12.022
28.
YangYLiXZhaoXLiuYZhaoTZhuQ. Long-Term Antibody Response to Inactivated SARS-CoV-2 Vaccination in Patients With Chronic Liver Disease: A Multicenter Study. Clin Res Hepatol Gastroenterol (2023) 47(7):102150. 10.1016/j.clinre.2023.102150
29.
WuSWangXFengMLiuXFanXRanXet alSafety and Immunogenicity of Inactivated COVID-19 Vaccine CoronaVac and the RBD-Dimer-Based COVID-19 Vaccine ZF2001 in Chronic Hepatitis B Patients. Front Med (Lausanne) (2023) 10:1078666. 10.3389/fmed.2023.1078666
30.
WilluweitKFreyAPassenbergMKorthJSakaNAnastasiouOEet alPatients With Liver Cirrhosis Show High Immunogenicity Upon COVID-19 Vaccination But Develop Premature Deterioration of Antibody Titers. Vaccines (Basel). (2022) 10(3):377. 10.3390/vaccines10030377
31.
WillauerANRousterSDMeedsHLJenningsCLAbdel-HameedEADariaDEet alHumoral and T-Cell-Mediated Immunity to SARS-CoV-2 Vaccination in Patients With Liver Disease and Transplant Recipients. Hepatol Commun (2023) 7(4):e0100. 10.1097/HC9.0000000000000100
32.
TiYNHanBLiuTFYuanYJZhangLY. Efficacy and Safety of Inactivated Novel Coronavirus Vaccine Inoculation in Patients With Chronic Hepatitis B. Zhonghua gan zang bing Za Zhi = Zhonghua ganzangbing zazhi = Chin J Hepatol (2022) 30(12):1370–4. 10.3760/cma.j.cn501113-20220825-00437
33.
SinghADeASinghMPRathiSVermaNPremkumarMet alAntibody Response and Safety of ChAdOx1-nCOV (Covishield) in Patients With Cirrhosis: A Cross-Sectional, Observational Study. Dig Dis Sci (2023) 68(2):676–84. 10.1007/s10620-022-07641-2
34.
LiuYLuJZhanHYuanWLiXKangHet alInactivated SARS-CoV-2 Booster Vaccine Enhanced Immune Responses in Patients With Chronic Liver Diseases. Virol Sin (2023) 38(5):723–34. 10.1016/j.virs.2023.07.005
35.
LiuFFengXDuJRuanMLiuH. Serologic Status and Safety of Inactivated COVID-19 Vaccine for Hepatocellular Carcinoma Patients With Cirrhosis After Curative Liver Resection. Cancer Commun (Lond) (2023) 43(3):409–12. 10.1002/cac2.12398
36.
LiHWangYAoLKeMChenZChenMet alAssociation Between Immunosuppressants and Poor Antibody Responses to SARS-CoV-2 Vaccines in Patients With Autoimmune Liver Diseases. Front Immunol (2022) 13:988004. 10.3389/fimmu.2022.988004
37.
LiHLiSXuPWangXDengHLeiYet alAnalysis of Neutralizing Antibodies to COVID-19 Inactivated or Subunit Recombinant Vaccines in Hospitalized Patients With Liver Dysfunction. Front Immunol (2023) 14:1084646. 10.3389/fimmu.2023.1084646
38.
KulkarniAVJaggaiahgariSIyengarSSimhadriVGujjarlapudiDRugwaniHet alPoor Immune Response to Coronavirus Disease Vaccines in Decompensated Cirrhosis Patients and Liver Transplant Recipients. Vaccine (2022) 40(48):6971–8. 10.1016/j.vaccine.2022.10.042
39.
IvashkinVIsmailovaADmitrievaKMaslennikovRZharkovaMAlievSet alEfficacy and Safety of COVID-19 Vaccination in Patients With Cirrhosis. World J Hepatol (2022) 14(7):1470–9. 10.4254/wjh.v14.i7.1470
40.
GoelAVermaATiwariPKatiyarHAggarwalAKhetanDet alSerological Immune Response Following ChAdOx1 nCoV-19 Vaccine (Covishield(®)) in Patients With Liver Cirrhosis. Vaccines (Basel) (2022) 10(11):1837. 10.3390/vaccines10111837
41.
DuengelhoefPHartlJRütherDSteinmannSBrehmTTWeltzschJPet alSARS-CoV-2 Vaccination Response in Patients With Autoimmune Hepatitis and Autoimmune Cholestatic Liver Disease. United Eur Gastroenterol J (2022) 10(3):319–29. 10.1002/ueg2.12218
42.
ChenZZhangYSongRWangLHuXLiHet alWaning Humoral Immune Responses to Inactivated SARS-CoV-2 Vaccines in Patients With Severe Liver Disease. Signal Transduct Target Ther (2022) 7(1):174. 10.1038/s41392-022-01032-9
43.
ChenZWangYHeTLiHAoLPanQet alSafety and Immunogenicity After Primary and Booster Inactivated SARS-Cov-2 Vaccination in Patients With Autoimmune Liver Diseases. J Clin Transl Hepatol (2024) 12(2):162–71. 10.14218/JCTH.2023.00049
44.
BiliottiECaioliASoraceCLionettiRMilozziETaibiCet alHumoral Immune Response After COVID-19 mRNA Vaccination in Patients With Liver Cirrhosis: A Prospective Real-Life Single Center Study. Biomedicines (2023) 11(5):1320. 10.3390/biomedicines11051320
45.
Al-DurySWaernJWaldenströmJAlavanjaMSaedHHTörnellAet alImpaired SARS-CoV-2-Specific T-Cell Reactivity in Patients With Cirrhosis Following mRNA COVID-19 Vaccination. JHEP Rep (2022) 4(7):100496. 10.1016/j.jhepr.2022.100496
46.
SchultzNHSorvollIHMichelsenAEMuntheLALund-JohansenFAhlenMTet alThrombosis and Thrombocytopenia After ChAdOx1 nCoV-19 Vaccination. N Engl J Med (2021) 384(22):2124–30. 10.1056/NEJMoa2104882
47.
OsterMEShayDKSuJRGeeJCreechCBBroderKRet alMyocarditis Cases Reported After mRNA-Based COVID-19 Vaccination in the US From December 2020 to August 2021. JAMA (2022) 327(4):331–40. 10.1001/jama.2021.24110
48.
CaoYYisimayiAJianFSongWXiaoTWangLet alImprinted SARS-CoV-2 Humoral Immunity Induces Convergent Omicron RBD Evolution. Nature (2022) 614:521–9. 10.1038/s41586-022-05644-7
Summary
Keywords
vaccine, meta-analysis, safety, efficacy, chronic liver disease
Citation
Xiao G, He T, Zhang B, Yang Z, Ling N, Chen M, Zhang D, Hu P, Zhang G, Peng M, Cai D and Ren H (2024) Safety and Efficacy of SARS-CoV-2 Vaccines in Patients With Chronic Liver Diseases: A Systematic Review and Meta-Analysis. Int J Public Health 69:1605295. doi: 10.3389/ijph.2024.1605295
Received
04 August 2022
Accepted
08 November 2024
Published
21 November 2024
Volume
69 - 2024
Edited by
Olaf von dem Knesebeck, University Medical Center Hamburg-Eppendorf, Germany
Reviewed by
Emmanuel Bhaskar, Sri Ramachandra University, India
Updates
Copyright
© 2024 Xiao, He, Zhang, Yang, Ling, Chen, Zhang, Hu, Zhang, Peng, Cai and Ren.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Ren, renhong0531@cqmu.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.