- 1School of Public Health of Inner Mongolia Medical University, Hohhot, China
- 2Division of Molecular Signaling, Department of the Advanced Biomedical Research, Interdisciplinary Graduate School of Medicine, University of Yamanashi, Kofu, Japan
Objective: Preterm birth (PTB) is considered as a public health problem and one of the main risk factors related to the global disease burden. The purpose of this study aims to explore the influence of exposure to major air pollutants at different pregnancies on PTB.
Methods: The relationship between air pollutants and PTB in China was collected from cohort studies and case-control studies published before 30 April 2022. Meta-analysis was carried out with STATA 15.0 software.
Results: A total of 2,115 papers were retrieved, of which 18 papers met the inclusion criteria. The comprehensive effect of pollutant exposure and PTB were calculated. PM2.5 during entire pregnancy and O3 exposure during third trimester were positively associated with preterm birth. Every 10 μg/m3 increase in the average concentration of PM2.5 during the whole pregnancy will increase the risk of premature delivery by 4%, and every 10 μg/m3 increase in the average concentration of O3 in the third trimester will increase the risk of premature delivery by 1%.
Conclusion: Exposure to PM2.5 entire prenatal pregnancy and O3 in third trimester is associated with an increased risk of preterm birth occurrence.
Introduction
Preterm birth (PTB) is defined as delivery before 37 weeks of pregnancy, which has long-term negative impacts on the development of newborns and may lead to stunting. PTB is becoming more and more common in developing and even some developed countries [1], although the underlying causes are still unknown. Premature delivery is a common adverse pregnancy outcome. Because of its serious impact on life and health, it brings a great burden on families and society, leading to perinatal and early neonatal death (especially in developing countries) and immature organ development, which leads to physical disability or mental disorders [2, 3]. PTB is regarded as a public health issue and one of the key risk factors contributing to the Global Burden of Disease. As a result, there is an urgent need to investigate PTB risk factors in order to reduce the occurrence of PTB and thus improve human health.
PTB has been linked to a variety of factors, including social and economic status, race, genetic influences, medical conditions, mental disorders, and environmental exposures [4]. Air pollution is a major component of environmental pollution, and it is considered as a risk factor of many diseases. According to the latest estimate of the World Health Organization, about 90% of the world’s population breathes air with high concentrations of pollutants, and as many as 7 million people die every year due to environmental and indoor air pollution [5]. The impact of air pollution on health has become a global public health problem (6). In addition to causing respiratory [7, 8] and cardiovascular diseases [9], more and more studies have investigated the influence of air pollution on premature delivery, including China [10], South Korea [11], Japan [12] and the United States [13]. Although most published studies have reported that various air pollutants are related to PTB, the nature of the pollutants studied and the related pregnancy outcomes are different. At present, there is a lack of toxicological data to guide pregnant women to choose the most relevant vulnerable exposure window [14]. Previous surveys have used a wide range of exposure windows (i.e., weeks, months and 3 months) to determine the time relationship between air pollution and adverse pregnancy outcomes [15]. Some studies have reported that exposure in the first trimester of pregnancy is associated with an increased risk of PTB [16]. Other studies have shown that exposure in the third trimester has a greater impact [17]. Therefore, the relationship between different pollutants exposure and different adverse pregnancy outcomes is complicated, and different periods of pollutant exposure during pregnancy have different effects on pregnancy outcomes [18]. Some previous studies have discussed the effect of air pollution on PTB [19–21], but they are mainly carried out in developed countries. Although the level of air pollution in Asian countries is usually higher, there has been a lack of research in these countries [10, 22]. Fetuses are particularly sensitive to environmental hazards because cell proliferation, differentiation and organ development accelerate during pregnancy, which may directly damage the fetus or interfere with placental function [23]. Several possible biological approaches have been put forward in previous studies. On the one hand, placental DNA adducts produced by air pollution are more common in mothers exposed to high levels of air pollution [24]. The high expression of fetal DNA adducts may reduce the ability of DNA repair and detoxification enzymes [25]. In previous studies, DNA adducts were associated with shortened pregnancy [26]. On the other hand, its mechanism may be related to hematological factors. The increase of blood viscosity caused by exposure to air pollution and hematological factors may be related to insufficient placental perfusion, which leads to PTB(24). Animal studies also support the finding that exposure to urban air pollution during pregnancy will inhibit fetal growth, which is closely related to the risk of premature delivery [27]. Therefore, this paper plans to collect the previous cohort and case-control studies, effectively increasing the sample size, and further improving the evidence level by including only the studies with high Newcastle-Ottawa Quality Score (NOS) and discussing the effects of air pollution exposure during different pregnancies on PTB.
Methods
Literature Search
Inclusion criteria: 1) published before 31 April 2022, the contents of the literature were all independent epidemiological findings; 2) the study was about the effect of atmospheric pollutants on preterm birth in China; 3) all studies defined delivery at less than 37 weeks of gestational age as preterm birth according to WHO criteria; and defined the 3 gestational window periods in the following way, namely: the first 3 months of pregnancy (before the end of 12 weeks of gestation) as early gestation, second trimester at the end of 13–27 weeks of gestation, and third trimester after 28 weeks of gestation; 4) the risk factors studied included air pollutants; 5) the results of each literature is the quantitative dose-response relationships between air pollution and premature delivery, including parameter estimation and 95% CI of the risk of premature delivery with the increase in air pollutant concentration of 10 μg/m3; 6) the study methods were cohort studies or case-control studies.
Exclusion criteria: 1) animal studies, in vitro toxicology studies, reviews and review articles; 2) studies with risk factors limited to indoor pollution, some kind of heavy metal or organic pollution; 3) studies examining the effects of air pollution due to occupational exposure on adverse pregnancy outcomes; 4) studies on the birth outcomes except premature delivery; 5) studies for which RR values and 95% CI were not available; 6) the original article contains no original data.
Literature Search Strategy
The literature on the effect of outdoor air pollution on preterm birth in China published at home and abroad before 31 April 2022, was collected by computer search of PubMed, Cochrane Library, Web of science, China Journal Full Text Database (CNKI), Wanfang Science and Technology Information Database and Weipu Chinese Science and Technology Journal Full Text Database, and by tracing the relevant literature. The retrieved keywords were: air pollution, environmental pollution, air quality, atmospheric pollution, atmospheric pollutants, PM10, PM2.5, NO2, SO2, O3, sulfur dioxide, nitrogen dioxide, ozone, particulate matter, adverse birth outcomes, adverse pregnancy outcomes, preterm birth premature birth, preterm delivery, small for gestational age, Chinese, China, atmospheric pollution, air pollution, particulate matter, respirable particulate matter, fine particulate matter, sulfur dioxide, nitrogen dioxide, ozone, adverse pregnancy outcomes, preterm birth, less than gestational age.
Literature Screening and Extraction
The literature search was conducted independently by two researchers, and the literature initially examined was screened one by one according to the inclusion and exclusion criteria, and relevant information was extracted and checked for differences. If they had different opinions, they all discussed and unified. The information extracted included authors, year of publication, study site, study duration, number of study participants, study method, exposure assessment method, type of air pollutant, and period of exposure. Because the sensitivity of fetuses to pollutant exposure in different periods is different, and the results of the influence of pollutant exposure on premature delivery in different periods may be different, most of the original documents are analyzed separately according to gestational period, so the data are extracted separately according to gestational period.
Statistical Analysis
Statistical software Stata 15.0 was used for meta-analysis of data, and I2 statistics were used to measure the heterogeneity of individual research results. If there was no heterogeneity between the studies (I2 < 50% or p > 0.10), the fixed effect model was used to calculate the comprehensive RR value and its 95% CI. If there is heterogeneity, a random effects model was used and subgroup analysis was used to explore the source of heterogeneity. Sensitivity analysis was used to evaluate the stability of the Meta-analysis findings and to determine the differences in the combined effect values before and after excluding a particular study. Egger linear regression analysis was used to analyze the publication bias, and the test level was bilateral α = 0.01.
Results
General Description
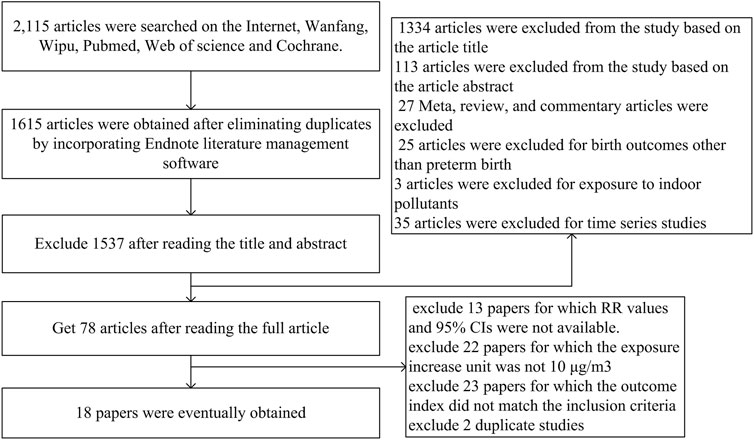
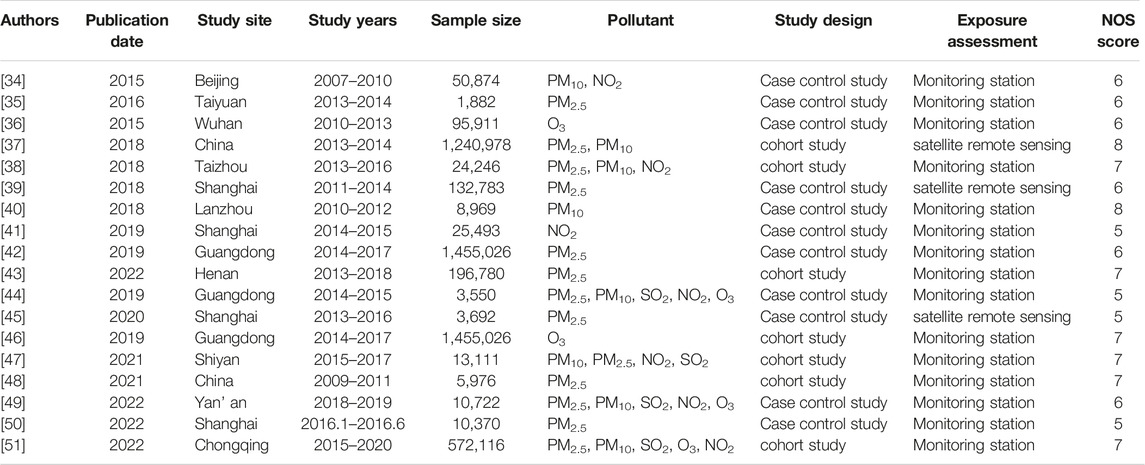
A total of 1,615 researches were retrieved. After a preliminary screening of the title and abstract, 78 potentially suitable studies were selected. Figure 1 depicts the flowchart of the study search and selection in the Meta-analysis and lists the 18 publications that were deemed eligible after additional assessment of the complete texts of these articles. The data features of the 18 papers included in this meta-analysis are shown in Table 1. In these 18 investigations, 11, 8, 4, 6 and 3 investigated the effects of PM2.5, PM10, SO2, NO2 and O3 on PTB respectively. Only two surveys have been conducted on Chinese national populations, most of which (n = 11) were conducted in southern cities, followed by northern cities (n = 5). There were about 1,882–1,240,978 participants in these studies. Three researchers used satellite data, and 15 researchers used monitoring station data to determine exposure assessment methods.
Heterogeneity Test
The results of exposure to PM2.5, PM10, SO2, NO2 and O3 in different pregnancy were analyzed by heterogeneity. The heterogeneity of O3 exposure results in the third trimester of pregnancy was low, and the effect is estimated by using fixed effect model, while the results of the other pollutants had high heterogeneity, the random effect model is used to the research results. The results showed that every 10 μg/m3 increase in PM2.5 concentration during the whole pregnancy increases the risk of PTB by 4% (RR: 1.04, 95% CI: 1.01, 1.07). The risk of PTB increased by 1% (RR: 1.01, 95% CI: 1.01, 1.02) for every 10 μg/m3 increase in O3 concentration in the third trimester. The effects of PM10, SO2 and NO2 exposure in different pregnancy on PTB were not found statistically significant. Table 2 and Figure 2 (Supplementary Figures S1–S3).
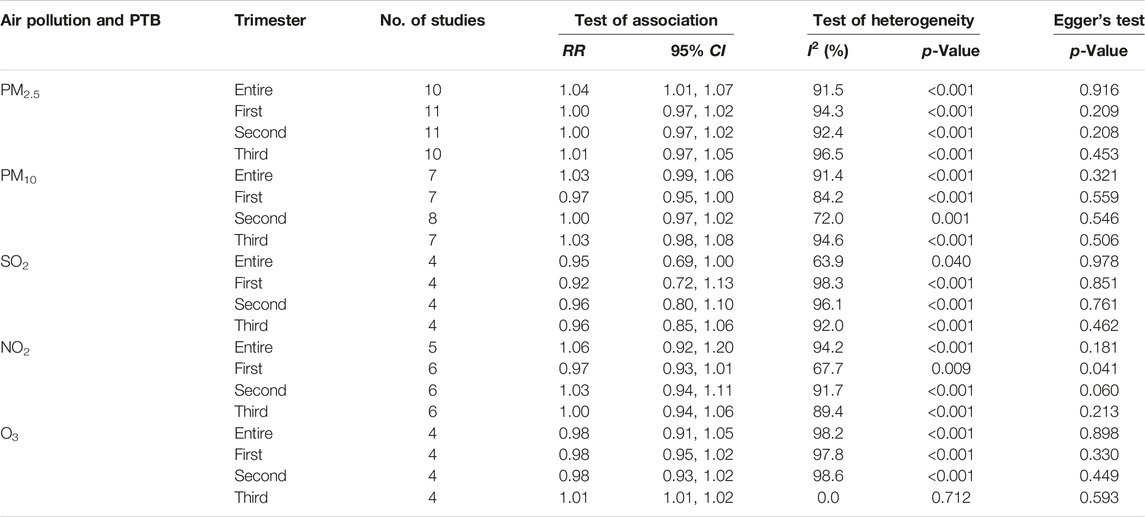
TABLE 2. Summary of integrated impact estimates of premature birth associated with air pollution (PM2.5, PM10, NO2, SO2 and O3) (China, 2023).
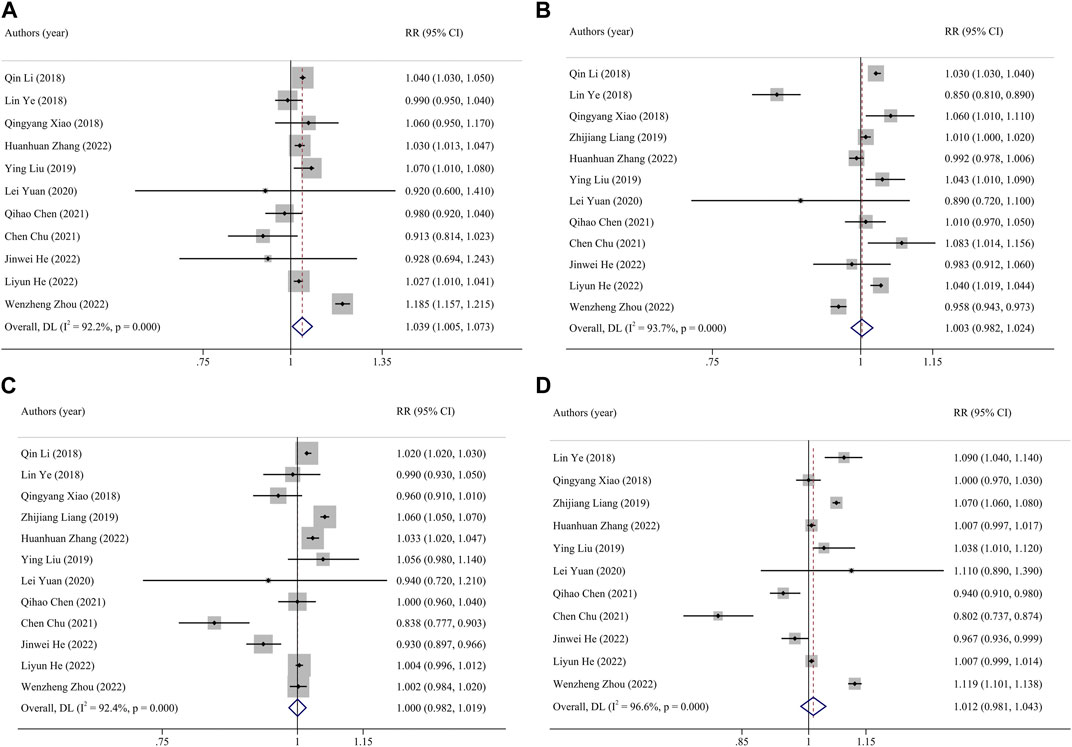
FIGURE 2. Funnel plot of the association between exposure to PM2.5 and premature birth. Pooled estimates of effect size are indicated by vertical points of diamonds, size of shaded area around the diamond is proportional to weight, and 95% CI are represented by horizontal line. ((A), entire pregnancy; (B), first trimester; (C), second trimester; (D), third trimester). (China, 2023).
Subgroup Analysis
By further analysis of different research methods (cohort and case-control study). In the case-control study, every 10 μg/m3 increase in PM2.5 concentration during the whole pregnancy increased the risk of PTB by 4% (RR:1.04, 95% CI: 1.01, 1.07). Every 10 μg/m3 increase in the average concentration of SO2 in early and late pregnancy will increase the risk of PTB by 4% (first pregnancy RR: 1.04, 95% CI: 1.02, 1.07, late pregnancy RR: 1.04, 95% CI: 1.01, 1.06). In the cohort study, the risk of PTB was reduced by 11%, 15%, and 12% for each 10 μg/m3 increase in SO2 concentration throughout pregnancy, mid-term and late in pregnancy (whole pregnancy RR: 0.89, 95% CI: 0.84, 0.94, second pregnancy RR: 0.85, 95% CI: 0.74, 0.97, late pregnancy RR: 0.88, 95% CI: 0.82). The risk of PTB was reduced by 6% for each 10 μg/m3 increase in NO2 concentration in first pregnancy (RR: 0.94, 95% CI: 0.90, 0.99). According to the study areas (southern cities, northern cities and the whole country), the influence of pollutant exposure on PTB was evaluated by stratified analysis. The results show that the influence of PM2.5 on PTB has statistical significance in the study of northern cities (RR: 1.03, 95% CI: 1.01, 1.05). Studies conducted in southern cities showed that the risk of PTB increased by 5% for every 10 μg/m3 increase in PM10 concentration during the whole pregnancy (RR: 1.05, 95% CI: 1.02, 1.09). Because the exposure concentration of pollutants may have different effects on health [28], we stratify the concentration of pollutants according to WHO standards. Stratified according to different concentrations of pollutants, when the concentration of PM2.5 exceeds 50 μg/m3, the PTB will increase by 2.5% (RR:1.025, 95% CI: 1.01, 1.04) for every 10 μg/m3 increase of PM2.5 during the whole pregnancy. 9% for every 10 μg/m3 increase of PM2.5 when the concentration is lower than 50 μg/m3. The heterogeneity of PM2.5 was significantly reduced in case-control study, northern city study and study with exposure concentration higher than 50 μg/m3. The heterogeneity of PM10 in southern cities, SO2 in cohort study and case-control study, and NO2 cohort study was significantly reduced. These may be the heterogeneous sources of merger effect. Table 3 and Figure 3.
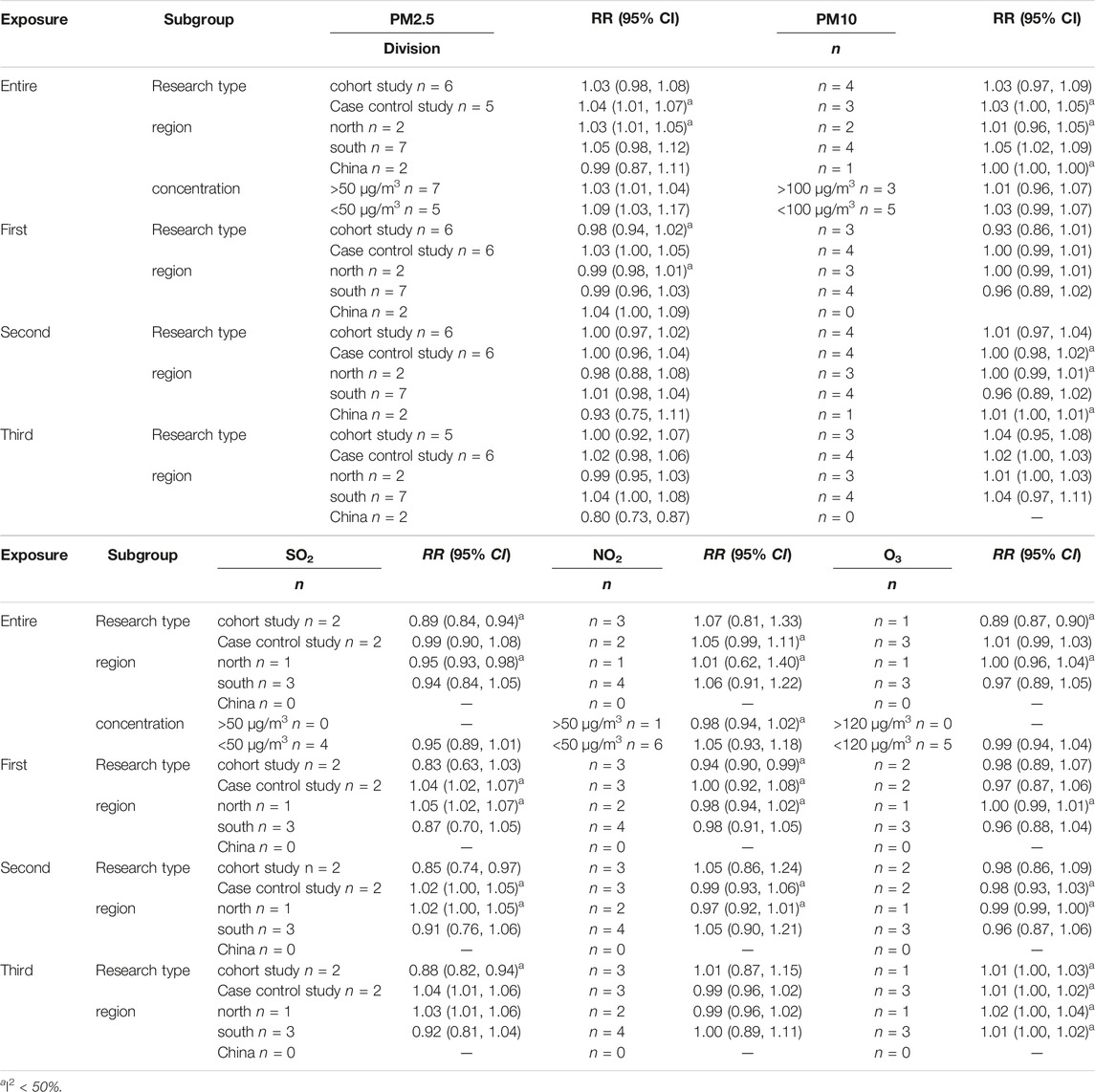
TABLE 3. Subgroup analysis of premature birth associated with air pollution (PM2.5, PM10, SO2, NO2 and O3) in different late gestational periods. (China, 2023).
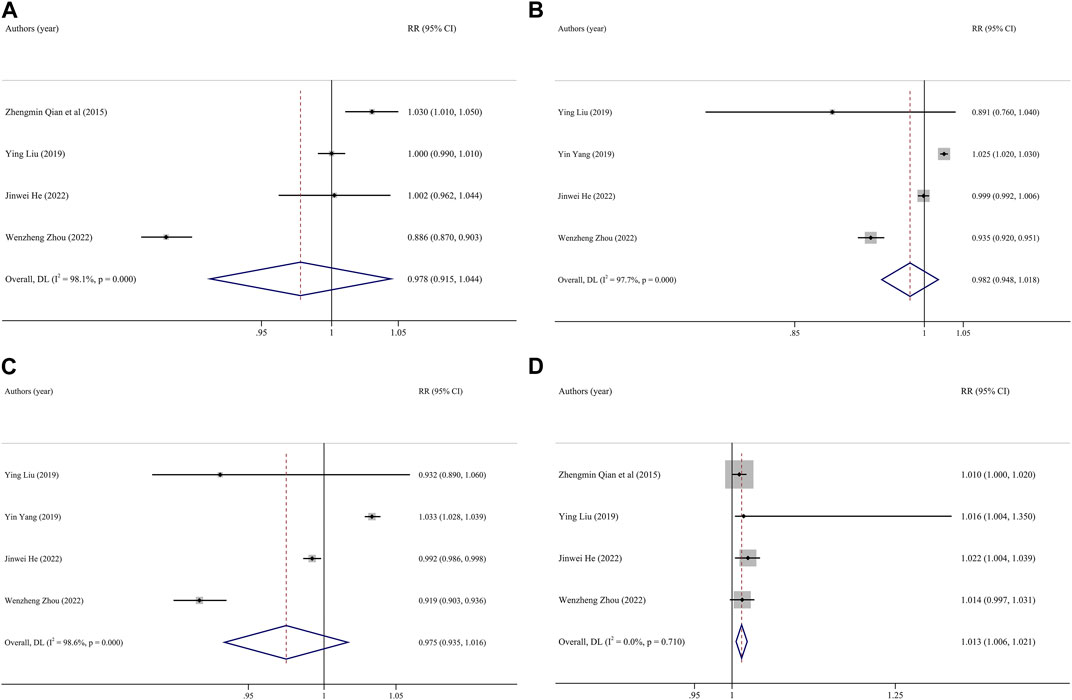
FIGURE 3. Funnel plot of the association between exposure to O3 and premature birth. Pooled estimates of effect size are indicated by vertical points of diamonds, size of shaded area around the diamond is proportional to weight, and 95% CI are represented by horizontal line. ((A), entire pregnancy; (B) first trimester; (C), second trimester; (D), third trimester). (China, 2023).
Publication Bias and Sensitivity Analysis
Taking lgRR as the abscissa and its standard deviation as the ordinate, the funnel diagram was drawn according to pregnancy periods, and it was found that there was no publication bias in the included literature. By excluding studies one by one in turn, the sensitivity analysis was conducted to evaluate the robustness of the effect, and it was found that the effect of various pollutants exposure on PTB risk were stable in this meta-analysis (Supplementary Figures S4–S25).
Discussion
The influence of air pollutants on premature delivery has become the focus of attention. This Meta-analysis included 18 eligible studies to investigate the correlation between the effects of each 10 μg/m3 increase in the mean concentration of five major ambient air pollutants (including PM2.5, PM 10, NO2, SO2 and O3) during pregnancy exposure on preterm birth. The results showed that exposure to PM2.5 throughout pregnancy and O3 in late pregnancy was positively correlated with PTB. Every 10 μg/m3 increase in the average concentration of PM2.5 in the whole pregnancy increased the risk of premature delivery by 4%, and every 10 μg/m3 increase in the average concentration of O3 in the third trimester increased the risk of premature delivery by 1%. Every increase of 10 μg/m3 in the exposure concentration of residual pollutants during different pregnancy has no significant effect on the incidence of premature delivery. As a major component of air pollution mixture, PM2.5 is more likely to harm human health due to its special characteristics, such as small size, large specific surface area, long floating time in the air, and adsorption capacity [29]. SUN, Chen et al. [29, 30] similarly found that PM2.5 exposure throughout pregnancy was associated with preterm delivery, and an increase of PM2.5 in early to mid-late pregnancy 10 μg/m3, the risk of preterm birth occurrence was not significant. Luo et al. [31] found that PM2.5 exposure throughout pregnancy was not associated with preterm birth. Liu et al. [32] reported results shows that there is a significant correlation between low exposure of PM2.5 in early pregnancy and premature delivery. This inconsistency may be due to the different number of studies involved due to different increments in pollutant exposure levels, search times, and search scopes, which may be the main reason for the discrepancy in study results. Current epidemiological evidence suggests that women in the first trimester of pregnancy tend to be less active outdoors when reported PM2.5 concentrations are high, which may overestimate ambient PM2.5 exposure and lead to bias. However, during periods of relatively low reported PM2.5 concentrations, they are relatively less aware of self-protection against air pollution, which may lead to a high risk of preterm birth.
Pregnancy is a time when people are most susceptible to the effects of air pollution, although past researches have produced mixed findings. Early gestation (first or third month), according to some studies, is the window of susceptibility, however later gestation is supported by other studies (third gestation, last month, or last week). In the present study, the effect of different gestational pollutant exposures on preterm birth was investigated and only O3 exposure in late pregnancy was found to promote preterm birth. The results of Ju et al. [33] also showed that O3 exposure in early and mid-late pregnancy was significantly associated with preterm birth. This is similar to our results, suggesting that the susceptibility window may be related to the specificity of different pollutants and needs to be further explored.
It is well known that the toxicity and health effects of air pollutants may vary by geographic region. Therefore, it is reasonable to assess the effects of different gestational air pollutant exposures on PTB by regional subgroup analysis. Subgroup analyses in different regions showed regional differences in the effects of air pollution on PTB. The results showed that the exposure of PM2.5 in the northern area and PM10 in southern area during pregnancy was significantly related to the increase of PTB, but other pollutants did not find this correlation. Due to the differences in living habits, climate and pollutant concentrations between the northern and southern regions, it was found that the concentration of PM2.5 in the northern region was higher than that in the southern region due to coal burning in winter. The quality of the results from long-term cohort studies is obviously better than that of case-control studies. Especially the prospective cohort studies, is considered to be the most reliable results in observational epidemiology. Therefore, we used different research methods to carry out subgroup analysis. The results showed that SO2 exposure in the second and third trimester of pregnancy was significantly correlated with the decrease of PTB, while in the case-control study, SO2 exposure in the first trimester of pregnancy was significantly correlated with the increase of PTB. The cohort study found that NO2 exposure in early pregnancy was significantly correlated with the decrease of PTB, while NO2 exposure in middle and late pregnancy was not significantly correlated with premature delivery.
We discovered substantial variability among the included studies, which is predictable in view of the limitations of our meta-analysis. In order to quantitatively aggregate individual estimates from studies with substantial heterogeneity, we employed a random effects model. Subgroup analysis was also used to investigate the causes of heterogeneity. The results show that, even though the study design and study area only partially explain the variability, most subgroup analyses still revealed significant heterogeneity. These results imply that due to the small number of related studies, variability of the included studies may be affected by other factors that are not considered in this analysis, such as socio-economic status and chemical composition of pollutants. Therefore, more studies are needed to investigate the causes of heterogeneity in the future. In several studies, personal samplers were used to measure the exposure of pregnant women to air pollutants, and the results were more accurate. Therefore, more research on personal information might be helpful for future analysis.
Conclusion
Exposure to PM2.5 throughout pregnancy may be related to the increased risk of PTB, but prenatal exposure to PM10, SO2, NO2 and O3 has no significant correlation. Analysis stratified by different gestational periods showed that O3 exposure in late pregnancy may increase the risk of preterm birth occurrence, but the overall level of effect was not high. The sensitivity windows for different pollutants are different, suggesting that more in-depth research is needed in this field in the future.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by the Inner Mongolia Autonomous Region (Health science and Technology Program) under grant (202201229) and the Inner Mongolia Medical University (“Zhiyuan Talents” project) under grant (ZY0201028).
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review, as well as the Inner Mongolia People’s Hospital, the Inner Mongolia Autonomous Region Health Committee Foundation Group, and Inner Mongolia Medical University for the “Zhiyuan Talents” project.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2023.1606226/full#supplementary-material
References
1. Beck, S, Wojdyla, D, Say, L, Betran, AP, Merialdi, M, Requejo, JH, et al. The Worldwide Incidence of Preterm Birth: A Systematic Review of Maternal Mortality and Morbidity. Bull World Health Organ (2010) 88(1):31–8. doi:10.2471/BLT.08.062554
2. Ngoc, NT, Merialdi, M, Abdel-Aleem, H, Carroli, G, Purwar, M, Zavaleta, N, et al. Causes of Stillbirths and Early Neonatal Deaths: Data From 7993 Pregnancies in Six Developing Countries. Bull World Health Organ (2006) 84(9):699–705. doi:10.2471/blt.05.027300
3. Lawn, JE, Gravett, MG, Nunes, TM, Rubens, CE, and Stanton, C, GAPPS Review Group. Global Report on Preterm Birth and Stillbirth (1 of 7): Definitions, Description of the burden and Opportunities to Improve Data. BMC pregnancy and childbirth (2010) 10:S1. doi:10.1186/1471-2393-10-S1-S1
4. Savitz, DA, Harmon, Q, Siega-Riz, AM, Herring, AH, Dole, N, and Thorp, JM. Behavioral Influences on Preterm Birth: Integrated Analysis of the Pregnancy, Infection, and Nutrition Study. Matern child Health J (2012) 16(6):1151–63. doi:10.1007/s10995-011-0895-5
5. WHO. Air Pollution. World Health Organization (2021). Available from: http://www.who.int/airpollution/en/ (Accessed January 13, 2021).
6. Brauer, M, Freedman, G, Frostad, J, van Donkelaar, A, Martin, RV, Dentener, F, et al. Ambient Air Pollution Exposure Estimation for the Global Burden of Disease 2013. Environ Sci Technol (2016) 50(1):79–88. doi:10.1021/acs.est.5b03709
7. Guan, WJ, Zheng, XY, Chung, KF, and Zhong, NS. Impact of Air Pollution on the Burden of Chronic Respiratory Diseases in China: Time for Urgent Action. Lancet (London, England) (2016) 388(10054):1939–51. doi:10.1016/S0140-6736(16)31597-5
8. Gouveia, N, and Junger, WL, ESCALA investigators. Effects of Air Pollution on Infant and Children Respiratory Mortality in Four Large Latin-American Cities. Environ Pollut (Barking, Essex: 1987) (2018) 232:385–91. doi:10.1016/j.envpol.2017.08.125
9. Soldevila Bacardit, N, Vinyoles Bargalló, E, Agudo Ugena, J, and Camps Vila, L. Air Pollution, Cardiovascular Risk and Hypertension. Hipertension y riesgo Vasc (2018) 35(4):177–84. doi:10.1016/j.hipert.2018.03.001
10. Li, X, Huang, S, Jiao, A, Yang, X, Yun, J, Wang, Y, et al. Association Between Ambient Fine Particulate Matter and Preterm Birth or Term Low Birth Weight: An Updated Systematic Review and Meta-Analysis. Environ Pollut (Barking, Essex : 1987) (2017) 227:596–605. doi:10.1016/j.envpol.2017.03.055
11. Seo, JH, Leem, JH, Ha, EH, Kim, OJ, Kim, BM, Lee, JY, et al. Population-Attributable Risk of Low Birthweight Related to PM10 Pollution in Seven Korean Cities. Paediatric perinatal Epidemiol (2010) 24(2):140–8. doi:10.1111/j.1365-3016.2009.01085.x
12. Kashima, S, Naruse, H, Yorifuji, T, Ohki, S, Murakoshi, T, Takao, S, et al. Residential Proximity to Heavy Traffic and Birth Weight in Shizuoka, Japan. Environ Res (2011) 111(3):377–87. doi:10.1016/j.envres.2011.02.005
13. Vinikoor-Imler, LC, Davis, JA, Meyer, RE, Messer, LC, and Luben, TJ. Associations Between Prenatal Exposure to Air Pollution, Small for Gestational Age, and Term Low Birthweight in a State-Wide Birth Cohort. Environ Res (2014) 132:132–9. doi:10.1016/j.envres.2014.03.040
14. Glinianaia, SV, Rankin, J, Bell, R, Pless-Mulloli, T, and Howel, D. Particulate Air Pollution and Fetal Health: A Systematic Review of the Epidemiologic Evidence. Epidemiology (2004) 15(1):36–45. doi:10.1097/01.ede.0000101023.41844.ac
15. Hansen, C, Neller, A, Williams, G, and Simpson, R. Low Levels of Ambient Air Pollution During Pregnancy and Fetal Growth Among Term Neonates in Brisbane, Australia. Environ Res (2007) 103(3):383–9. doi:10.1016/j.envres.2006.06.010
16. Bobak, M. Outdoor Air Pollution, Low Birth Weight, and Prematurity. Environ Health Perspect (2000) 108(2):173–6. doi:10.1289/ehp.00108173
17. Ritz, B, and Yu, F. The Effect of Ambient Carbon Monoxide on Low Birth Weight Among Children Born in Southern California Between 1989 and 1993. Environ Health Perspect (1999) 107(1):17–25. doi:10.1289/ehp.9910717
18. Woodruff, TJ, Parker, JD, Darrow, LA, Slama, R, Bell, ML, Choi, H, et al. Methodological Issues in Studies of Air Pollution and Reproductive Health. Environ Res (2009) 109(3):311–20. doi:10.1016/j.envres.2008.12.012
19. Malley, CS, Kuylenstierna, JC, Vallack, HW, Henze, DK, Blencowe, H, and Ashmore, MR. Preterm Birth Associated With Maternal Fine Particulate Matter Exposure: A Global, Regional and National Assessment. Environ Int (2017) 101:173–82. doi:10.1016/j.envint.2017.01.023
20. Johnson, S, Bobb, JF, Ito, K, Savitz, DA, Elston, B, Shmool, JL, et al. Ambient Fine Particulate Matter, Nitrogen Dioxide, and Preterm Birth in New York City. Environ Health Perspect (2016) 124(8):1283–90. doi:10.1289/ehp.1510266
21. Huang, H, Woodruff, TJ, Baer, RJ, Bangia, K, August, LM, Jellife-Palowski, LL, et al. Investigation of Association Between Environmental and Socioeconomic Factors and Preterm Birth in California. Environ Int (2018) 121(2):1066–78. doi:10.1016/j.envint.2018.07.027
22. Yang, J, and Zhang, B. Air Pollution and Healthcare Expenditure: Implication for the Benefit of Air Pollution Control in China. Environ Int (2018) 120:443–55. doi:10.1016/j.envint.2018.08.011
23. Selevan, SG, Kimmel, CA, and Mendola, P. Identifying Critical Windows of Exposure for Children's Health. Environ Health Perspect (2000) 108:451–5. doi:10.1289/ehp.00108s3451
24. Kannan, S, Misra, DP, Dvonch, JT, and Krishnakumar, A. Exposures to Airborne Particulate Matter and Adverse Perinatal Outcomes: A Biologically Plausible Mechanistic Framework for Exploring Potential Effect Modification by Nutrition. Environ Health Perspect (2006) 114(11):1636–42. doi:10.1289/ehp.9081
25. Myllynen, P, Pasanen, M, and Pelkonen, O. Human Placenta: A Human Organ for Developmental Toxicology Research and Biomonitoring. Placenta (2005) 26(5):361–71. doi:10.1016/j.placenta.2004.09.006
26. Liu, S, Krewski, D, Shi, Y, Chen, Y, and Burnett, RT. Association Between Gaseous Ambient Air Pollutants and Adverse Pregnancy Outcomes in Vancouver, Canada. Environ Health Perspect (2003) 111(14):1773–8. doi:10.1289/ehp.6251
27. Veras, MM, Damaceno-Rodrigues, NR, Caldini, EG, Maciel Ribeiro, AA, Mayhew, TM, Saldiva, PH, et al. Particulate Urban Air Pollution Affects the Functional Morphology of Mouse Placenta. Biol Reprod (2008) 79(3):578–84. doi:10.1095/biolreprod.108.069591
28. Apte, JS, Marshall, JD, Cohen, AJ, and Brauer, M. Addressing Global Mortality From Ambient PM2.5. Environ Sci Technol (2015) 49:8057–66. doi:10.1021/acs.est.5b01236
29. Sun, X, Luo, X, Zhao, C, Chung Ng, RW, Lim, CE, Zhang, B, et al. The Association Between Fine Particulate Matter Exposure During Pregnancy and Preterm Birth: A Meta-Analysis. BMC pregnancy and childbirth (2015) 15:300. doi:10.1186/s12884-015-0738-2
30. Chen, Y, Bing, MB, Zhao, YL, Yang, JM, Tao, YL, and Yan, H. Prenatal Exposure to Outdoor Air Pollution and Preterm Birth: A Meta-Analysis. Chin J Epidemiol (2016) 37(6):880–5. doi:10.3760/cma.j.issn.0254-6450.2016.06.028
31. Luo, D, Kuang, T, Chen, YX, Huang, YH, Zhang, H, and Xia, YY. Air Pollution and Pregnancy Outcomes Based on Exposure Evaluation Using a Land Use Regression Model: A Systematic Review. Taiwanese J Obstet Gynecol (2021) 60(2):193–215. doi:10.1016/j.tjog.2021.01.004
32. Liu, C, Sun, J, Liu, Y, Liang, H, Wang, M, Wang, C, et al. Different Exposure Levels of Fine Particulate Matter and Preterm Birth: A Meta-Analysis Based on Cohort Studies. Environ Sci Pollut Res Int (2017) 24(22):17976–84. doi:10.1007/s11356-017-9363-0
33. Ju, L, Li, C, Yang, M, Sun, S, Zhang, Q, Cao, J, et al. Maternal Air Pollution Exposure Increases the Risk of Preterm Birth: Evidence From the Meta-Analysis of Cohort Studies. Environ Res (2021) 202:111654. doi:10.1016/j.envres.2021.111654
34. Huang, C, Nichols, C, Liu, Y, Zhang, Y, Liu, X, Gao, S, et al. Ambient Air Pollution and Adverse Birth Outcomes: A Natural Experiment Study. Popul Health metrics (2015) 13:17. doi:10.1186/s12963-015-0050-4
35. Zhang, YW, Cheng, YP, Feng, YL, Zhao, L, Wang, J, Li, CX, et al. Study on PM2.5 Exposure of Atmospheric Fine Particles During Pregnancy and Its Influence on Premature Delivery. Chin J Epidemiol (2016) 37(4).
36. Qian, Z, Liang, S, Yang, S, Trevathan, E, Huang, Z, Yang, R, et al. Ambient Air Pollution and Preterm Birth: A Prospective Birth Cohort Study in Wuhan, China. Int J Hyg Environ Health (2016) 219(2):195–203. doi:10.1016/j.ijheh.2015.11.003
37. Li, Q, Wang, YY, Guo, Y, Zhou, H, Wang, X, Wang, Q, et al. Effect of Airborne Particulate Matter of 2.5 μm or Less on Preterm Birth: A National Birth Cohort Study in China. Environ Int (2018) 121(2):1128–36. doi:10.1016/j.envint.2018.10.025
38. Ye, L, Ji, Y, Lv, W, Zhu, Y, Lu, C, Xu, B, et al. Associations Between Maternal Exposure to Air Pollution and Birth Outcomes: A Retrospective Cohort Study in Taizhou, China. BMC pregnancy and childbirth (2018) 25(22):21927–36. doi:10.1007/s11356-018-1944-z
39. Xiao, Q, Chen, H, Strickland, MJ, Kan, H, Chang, HH, Klein, M, et al. Associations Between Birth Outcomes and Maternal PM(2.5) Exposure in Shanghai: A Comparison of Three Exposure Assessment Approaches. Environ Int (2018) 117:226–36. doi:10.1016/j.envint.2018.04.050
40. Zhang, YQ. Study on the Relationship Between Atmospheric Particulate Matter and Adverse Birth Outcome and Health Risk Assessment [Ph.D.]. Lanzhou: Lanzhou University. (2018).
41. Ji, X, Meng, X, Liu, C, Chen, R, Ge, Y, Kan, L, et al. Nitrogen Dioxide Air Pollution and Preterm Birth in Shanghai, China. Environ Res (2019) 169:79–85. doi:10.1016/j.envres.2018.11.007
42. Liang, Z, Yang, Y, Qian, Z, Ruan, Z, Chang, J, Vaughn, MG, et al. Ambient PM(2.5) and Birth Outcomes: Estimating the Association and Attributable Risk Using a Birth Cohort Study in Nine Chinese Cities. Environ Int (2019) 126:329–35. doi:10.1016/j.envint.2019.02.017
43. Zhang, H, Zhang, X, Zhang, H, Luo, H, Feng, Y, Wang, J, et al. Assessing the Effect of Fine Particulate Matter on Adverse Birth Outcomes in Huai River Basin, Henan, China, 2013-2018. Environ Pollut (Barking, Essex: 1987) (2022) 306:119357. doi:10.1016/j.envpol.2022.119357
44. Liu, Y, Xu, J, Chen, D, Sun, P, and Ma, X. The Association Between Air Pollution and Preterm Birth and Low Birth Weight in Guangdong, China. BMC public health (2019) 19(1):3. doi:10.1186/s12889-018-6307-7
45. Yuan, L, Zhang, Y, Wang, W, Chen, R, Liu, Y, Liu, C, et al. Critical Windows for Maternal Fine Particulate Matter Exposure and Adverse Birth Outcomes: The Shanghai Birth Cohort Study. Chemosphere (2020) 240:124904. doi:10.1016/j.chemosphere.2019.124904
46. Yang, Y, Liang, Z, Ruan, Z, Zhang, S, Zhao, Q, and Lin, H. Estimating the Attributable Burden of Preterm Birth and Low Birth Weight Due to Maternal Ozone Exposure in Nine Chinese Cities. Atmos Environ (2020) 222:117169. doi:10.1016/j.atmosenv.2019.117169
47. Chen, Q, Ren, Z, Liu, Y, Qiu, Y, Yang, H, Zhou, Y, et al. The Association Between Preterm Birth and Ambient Air Pollution Exposure in Shiyan, China, 2015-2017. Int J Environ Res Public Health (2021) 18(8):4326. doi:10.3390/ijerph18084326
48. Chu, C, Zhu, Y, Liu, C, Chen, R, Yan, Y, Ren, Y, et al. Ambient Fine Particulate Matter Air Pollution and the Risk of Preterm Birth: A Multicenter Birth Cohort Study in China. Environ Pollut (Barking, Essex: 1987) (2021) 287:117629. doi:10.1016/j.envpol.2021.117629
49. He, J, Cao, N, Hei, J, Wang, H, He, J, Liu, Y, et al. Relationship Between Ambient Air Pollution and Preterm Birth: A Retrospective Birth Cohort Study in Yan'an, China. Environ Sci Pollut Res Int (2022) 29:73271–81. doi:10.1007/s11356-022-20852-4
50. Zhu, LP, He, LY, Zhu, YX, Du, L, and Chen, RJ. Study on the Correlation Between Pregnancy Exposure of Fine Particulate Matter (PM_(2.5)) and Premature Delivery in Shanghai. Electron J Develop Med (2022) 10(2), 120–125.
Keywords: atmospheric pollutants, preterm birth, meta-analysis, China, adverse pregnancy outcome
Citation: Wang X, Wang X, Gao C, Xu X, Li L, Liu Y, Li Z, Xia Y and Fang X (2023) Relationship Between Outdoor Air Pollutant Exposure and Premature Delivery in China- Systematic Review and Meta-Analysis. Int J Public Health 68:1606226. doi: 10.3389/ijph.2023.1606226
Received: 22 May 2023; Accepted: 28 September 2023;
Published: 09 October 2023.
Edited by:
Gabriel Gulis, University of Southern Denmark, DenmarkReviewed by:
Santu Ghosh, St John’s Medical College, IndiaCopyright © 2023 Wang, Wang, Gao, Xu, Li, Liu, Li, Xia and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Fang, MTg2ODYwNjYxNzlAMTYzLmNvbQ==
†These authors have contributed equally to this work
This Review is part of the IJPH Special Issue “Public Health and Primary Care, is 1 + 1 = 1?”
 Xue Wang
Xue Wang Xin Wang2†
Xin Wang2†