Abstract
Objectives: We examined the short-term impact of the Smoking Ban Law (SBL) enacted in Chile in 2013 on low birth weight (LBW) rates in cities and its differential effects by different maternal age groups and city density.
Methods: We included 885,880 live births from 21 Chilean cities of ≥100,000 inhabitants. We examined the smoking and LBW prevalence distribution before and after the SBL. Through Poisson mixed effect models, we determined whether a meaningful change in LBW rate occurred after SBL implementation in the whole sample and stratified by city population density and maternal age group.
Results: LBW prevalence remained stable before and after the SBL implementation (6.1% and 6.3%, respectively), while women’s smoking prevalence had a relative reduction of 25.9% (p < 0.00001). No significant changes in LBW rate occurred after the implementation of SBL in the total sample or stratified by city density tertiles or maternal age groups.
Conclusion: SBL implementation did not show short-term impact on LBW rate in Chile. Further studies need to examine long-term impact of SBL on low birthweight.
Introduction
More than 20 million newborns with low birth weight (LBW, defined as birthweight <2,500 gr) occur worldwide each year, which is associated with detrimental effects on infant development and chronic illness during adulthood [1]. The prevalence of LBW in the population is made up of term newborns who, because of fetal growth retardation, are small for their gestational age (SGA) and pre-term newborns, whose birth weight might be adequate for their gestational age or are also SGA. Maternal smoking and exposure to second-hand smoking during pregnancy have been associated with fetal growth retardation, reducing mean birthweight and increasing the risk of LBW in the offspring [2–6].
In Chile, the tobacco burden is the highest among Latin America with a current smoking prevalence of 33% [7]; however, the smoking prevalence among women is currently lower compared to previous years. Data from National Health Survey showed that in 2003 smoking prevalence among women of all ages was 38,3%, while this prevalence was significantly lower at 29,1% in 2016–2017 [8, 9]. Smoking prevalence was higher among the population aged 20–29 years old but experienced a steep decrease between 2003 and 2016–2017 (from 60.5% to 41.1%) [9]. Part of the decrease in smoking prevalence particularly among the young population, and women, could be ascribed to the implementation of strong and progressively tobacco smoking banning nationwide. In 1995 the tobacco law was enacted (Law No. 19419), which restricted the consumption, sale, and advertising of products made with tobacco in Chile [10]. In March 2013, a new law (No. 20660) increased these restrictions by prohibiting smoking in closed places accessible to the public or for collective commercial use, on terraces that are not outdoors or have a roof that is attached to a wall and in sports venues, stadiums, or gyms. Additionally, this law forbade the sale of cigarettes within a radius of 100 m around schools, the sale or free delivery of cigarettes in units or loose, and in packages containing quantities of less than 10 units, all kinds of tobacco advertising, smoking in programs and advertising on TV shows broadcasted during minors’ hours. This law was enforced almost immediately and simultaneously across the nation, and it also regulated tobacco labeling [11].
Similar smoking ban law (SBL) implementations in other parts of the world (United States, China, Australia, United Kingdom) have shown to have a greater impact in reducing smoking among young people, possibly due to a delay in smoking initiation habits at early ages [12–15].
Other studies have shown that the effectiveness of SBL in reducing tobacco behavior in the population could be modified by urban environments. With a higher number of public places for social interaction, more dense cities have experienced a greater reduction in second-hand smoking than less dense cities [16, 17]. Some studies, the majority in high-income countries, have assessed the impact of SBLs on reducing LBW over the years. However, no study has examined variations in the impact of SBLs among women in different age groups or across cities with different population density [18–20].
In Latin America and the Caribbean (LAC), smoking policies have been implemented in 23 of 35 countries [21]. There are still countries that do not have regulation for smoking in public places and these have a higher prevalence of LBW in the population [21]. Knowing whether SBL contribute to reducing LBW prevalence in the short run could support the continuation of smoking restrictions in Chile, and the promotion of SBL implementation in other countries from LAC and the Global South that have not yet implemented public smoking restrictions.
This study aimed to examine whether implementation of SBL had an impact on the LBW rate and whether this effect is different among women of different age groups and cities with different population density. We hypothesized that SBL implementation contributed to reducing LBW prevalence, and that this effect is more significant among younger women and in more dense cities.
Methods
Study Setting and Data Sources
We performed an ecological analysis using data from SALURBAL study, which has compiled data for live births, population, and health from 371 cities with ≥100,000 inhabitants across 11 Latin-American countries (Argentina, Brazil, Chile, Colombia, Costa Rica, El Salvador, Guatemala, Mexico, Nicaragua, Panama, and Peru) [22]. Each city was defined geographically by administrative units (municipios) that encompassed the urban extent of the city in 2010 using satellite imagery [22]. For this analysis we used individual data from live birth registries linked to cities by the maternal place of residence and population projection from census for each city in Chile between 2011 and 2015 (n = 21 cities). We also used Census and National Health Survey databases for collection of sociodemographic and smoking prevalence, which data collection methods has been described before [22].
Intervention/Exposure
SBL was implemented almost immediately and simultaneously across the nation in March 2013. We then defined exposure as at least a complete month of having received the intervention. Following this rationale, the pre-intervention period was considered from January 2011 to March 2013 and the post-intervention period from April 2013 to December 2015.
Sample
We included all live births that occurred during the two above mentioned periods in all cities with ≥100,000 inhabitants (n = 21) from Chile. Figure 1 shows the sample selection flowchart.
FIGURE 1
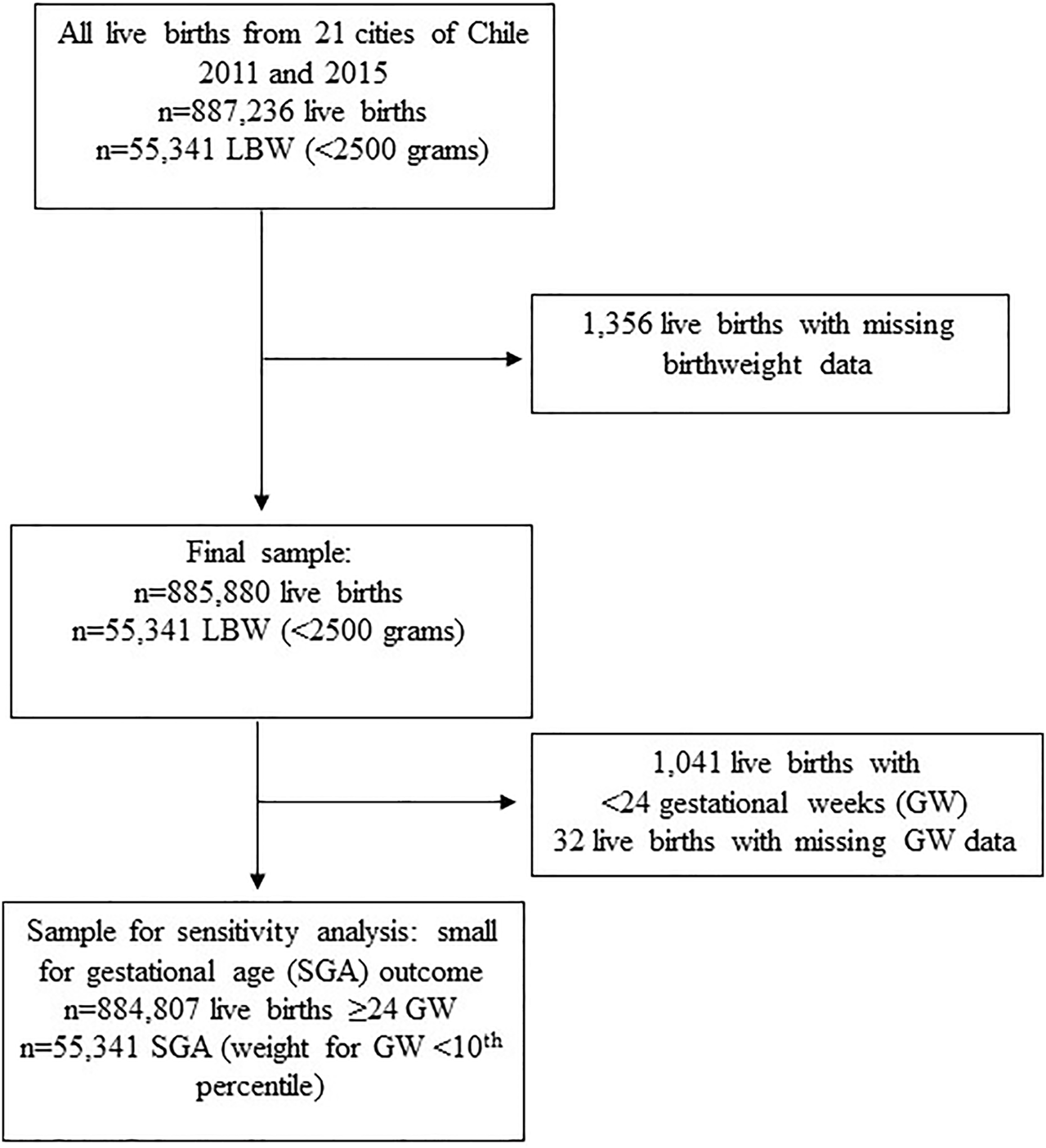
Sample selection flowchart. LBW: Low birth weight (birthweight <2,500 g), Chile, 2011–2015.
Outcome
LBW rate was defined as the number of newborns with birthweight less than 2.5 kg per 1,000 live births in each city per trimester during January 2011- March 2013 (pre-intervention period, 9 trimesters) and during April 2013–December 2015 (post-intervention period, 11 trimesters).
Other Variables
We included in our analysis variables that are shown to be related with the prevalence of LBW and with smoking behavior in the population.
Maternal age groups: categorized as <20 years old, 20–34 years old, and ≥35 years old.
Population density: To determine city population density, we calculated the mean city density for the period between 2011 and 2015 for each city, which was calculated as the number of people living per km2 of built-up area within the geographic boundaries of the municipios [22]. Distribution of cities countrywide by population density categories is shown in Supplementary Figure S1.
Smoking prevalence: we determined the prevalence of current smokers in cities by sex and age groups using National Health Survey databases (2009–2010 and 2016–2017). Current smokers were defined as those who smoked daily (≥1 cigarette/day) or occasionally (<1 cigarette/day) in the past 6 months.
Statistical Analysis
We described trends in LBW and smoking prevalence among women, by tertiles of city population density, and maternal age groups over the pre- and post-intervention period. We used a mixed effects analysis of variance (ANOVA) model to compare changes on smoking prevalence before and after the SBL implementation (time effect within cities) and differences between tertiles of population density and maternal age categories. Regression discontinuity was used to determine whether a change in the prevalence of LBW occurred immediately after Chile passed the SBL. Regression discontinuity analysis allows the assessment of the average treatment effect of an intervention by comparing observations or trends closely before and after the implementation of such intervention [23]. The selection of a short time-frame was used to reduce possible bias due to the effect of other interventions or other time-variant exposures on LBW rate.
We examined whether changes in LBW rate were associated with SBL intervention through Poisson mixed effect models. We accounted for city clustering by including a random intercept for cities. The time unit was defined as calendar trimesters as shown in the formula below.
denotes the rate of LBW in trimester t and city i. We considered
tas the number of trimesters, where t = 0 corresponds to the first trimester of the whole period. The period before the implementation of the smoking ban corresponds to 9 trimesters with
t≥0 and ≤8, while the ‘post-intervention’ corresponds to 11 trimesters with t > 8. X is a dummy variable that takes the value 0 in the pre-intervention period and 1 in the post-intervention period. Our parameters of interest were:
• β1 captures the change in LBW associated with a time unit increase (i.e., change in slope) in the period before the SBL (pre-intervention trend).
• β2 captures the level change in the LBW immediately after the SBL becomes effective, which ultimately corresponds to the “gap” between the pre and post intervention trends.
• β3 as it indicates the change in the temporal trend (i.e., change in slope) after the intervention.
If the smoking ban legislation has an immediate reduction in the rate of LBW, the coefficient β2 should be a negative value and significantly different from zero. If SBL has, on the opposite, a gradual influence in the reduction of LBW rate we will find that β3 coefficient will be of negative value and significantly different from zero.
For assessing differential effects by maternal age and city density, we stratified the main analysis by maternal age groups and by tertiles of city population density. We also performed a second model adjusted by seasonality using quarters of each year as dummy variables (1 = January–March, 2 = April–June, 3 = July–September, 4 = October–December).
We performed multiple sensitivity analyses. Firstly, to account for a possible delay in the effect of the policy due to the inclusion of live births that may have been exposed to “pre – intervention” smoking law conditions for some part of their gestation, we added lags at 1, 2, and 3 calendar trimesters (3, 6, and 9 months) after the intervention using the main analytical approach (trimesters cut-off points: July 2013, October 2013, January 2014, respectively). Secondly, we used a more refined time analysis where we calculated and modeled monthly rates of LBW (instead of calendar trimesters) from January 2012 to December 2013 to estimate the same coefficients as in the main model. Third, we analyzed LBW rate as a continuous outcome using mixed effects models. As total live births varied across cities affecting the LBW rate, we considered weights in the models (weight = √number of live births) for each city. Lastly, we carried out the main analytical strategy only including LBW infants who had a diagnosis of small for gestational age (SGA) among newborns with ≥24 GW. We defined SGA as those with birthweight lower than the 101h percentile according to Alarcón- Pittaluga curves built for Chilean newborns older than 24 GW [24].
Results
Table 1 shows baseline and relative changes in live birth, maternal, and city characteristics by tertiles of population density for the pre and post intervention periods for all 21 cities. Overall, baseline LBW prevalence was 6.1%. Before SBL implementation, cities with medium- density (Tertile 2 = 6,198–8,132 persons/km2) had a lower LBW prevalence compared to those with the lowest and highest density (Tertiles 1 and 3). Overall baseline prevalence of preterm newborns (<37 GW) was 7.8% and high- density cities (Tertile 3 ≥8,134 persons/km2) had a higher rate of preterm births compared to those with lower density. LBW prevalence remained quite stable over time, with a slight increase during the post-intervention period at the expense of pre-term newborns and in cities with medium density (Tertile 2, Table 1).
TABLE 1
| Births before smoking ban law (January 2011-March 2013) | Relative change between before and after the implementation of SBL | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 398,101) | Tertile 1 (n = 44,482) | Tertile 2 (n = 76,752) | Tertile 3 (n = 276,867) | Total (n = 487,779) | Tertile 1(n = 54,844) | Tertile 2 (n = 93,175) | Tertile 3 (n = 339,760) | |
| Live birth characteristics | ||||||||
| Total LBW, n (%) | 24,447 (6.1%) | 2,801 (6.3%) | 2,624 (5.7%) | 17,293 (6.3%) | 3.3% | 3.2% | 5.3% | 1.6% |
| LBW ≥37 GW, n(%) | 6,493 (1.8%) | 862 (2.1%) | 1,151 (1.6%) | 4,480 (1.8%) | 0% | 4.8% | 0% | 0% |
| <37 GW, n(%) | 31,066 (7.8%) | 3,159 (7.1%) | 5,529 (7.2%) | 22378 (8.1%) | 3.8% | 5.6% | 8.3% | 2.5% |
| Maternal characteristics | ||||||||
| <19 years old, n(%) | 54,135 (14%) | 6,614 (15%) | 11,091 (14%) | 36,430 (13%) | −21.40% | −20.0% | −14.3% | −15.4% |
| 20–34 years old, n(%) | 275,730 (69%) | 30,947 (70%) | 53,062 (69%) | 191,721 (69%) | 2.9% | 1.4% | 2.9% | 2.9% |
| ≥35 years old, n(%) | 68,236 (17%) | 30,947 (16%) | 12,599 (16%) | 48,716 (18%) | 5.9% | 6.3% | 6.3% | 0.0% |
| Maternal education ≥12 years, n(%) | 302,961 (76%) | 32,616 (73%) | 56,840 (74%) | 213,505 (77%) | 5.3% | 5.5% | 5.4% | 3.9% |
| City characteristics | ||||||||
| City density, inhabitants/km2 | 7,238 (1805) | 5,605 (357) | 6,704 (674) | 9,405 (1,164) | 3.2% | 3.1% | 3% | 3% |
| City population (inhabitants), p50 (IQR) | 216,138 (188,751) | 154,913 (129,441) | 213,212 (253,701) | 348,604 (722,791) | 3.5% | 3.2% | 3% | 4% |
| Women at reproductive age, % (min-max) | 52.6% (48.1–55.5) | 52.0% (48.1–53.7) | 52.8% (50.2–55.5) | 53.1 (50.6–54.6) | −1.3% | −1.5% | −1.5% | −1.1% |
| General fertility rate, (min-max) | 56.9 (49.5–71.6) | 58.3 (50.9–69.3) | 54.5 (49.5–59.7) | 57.8 (49.5–71.6) | −2.5% | −1.0% | −3.1% | −3.1% |
| Overall Smoking prevalence, mean (min-max) | 39.3% (33.9–46.3) | 38.8% (35.2–42) | 40.2% (38.5–45.4) | 38.9% (33.9–46.3) | −18.8%a | −13.7%a | −21.1%a | −21.6%a |
| Smoking prevalence in women, mean (min-max) | 37.8% (30.6–44.7) | 37.6% (30.6–43.1) | 38.6% (33.6–44.7) | 37.1% (31.9–40.9) | −25.9%a | −17.6%a | −32.1%a,b | −27.8%a |
| W 20–34 years old | 45.8% (38.7–53.3) | 46.2% (39.5–53.3) | 47.0% (41.4–52.7) | 44.1% (38.7–50.9) | −30.1%a | −24.5%a | −35.5%a,b | −30.4%a |
| W 35–49 years old | 39.6% (32.8–47.0) | 40.0% (33.6–47.0) | 40.7% (35.3–46.3) | 38.0% (32.8–44.5) | −26.5%a | −20.5%a | −32.4%a,b | −26.6%a |
Maternal and live birth characteristics before and after the implementation of the smoking ban law by tertiles of city density in law in all 21 cities of 100K+ residents in Chile, 2011–2015.
Indicates a significant change after the SBL implementation (p < 0.05).
Indicates significant difference with tertile 1 (p < 0.05). Relative change was calculated as the difference between mean, p50 or prevalence (categorical variables) before and after the SBL (Relative change = post-pre)/pre).
SBL, smoking ban law; LBW, Low birth weight (birthweight <2,500 g); GW, gestational weeks at birth, Women at reproductive age: proportion of women between 15 and 49 years old/total of women population; General fertility rate: number of live births per 1,000 women at reproductive age (15–49 years old). Categoric variables are expressed in frequency (n) and prevalence (%). Continuous variables are expressed in average and standard deviation or median (p50) and interquartile range (IQR) when indicated.
Before the implementation of SBL, overall smoking prevalence was similar across city density tertiles (37.1%–38.6%). Women aged 20–34 years had a higher smoking prevalence compared to those ≥35 years old at all tertiles for population density (p < 0.0001). Smoking prevalence had a relative decrease of 18.8% in the overall population after SBL with a higher decrease in women compared to men (−25.9% vs. −12.2%, respectively; p = 0.04). Mid-density cities had a higher decrease compared to lower-density cities (−32.1% vs. −17.6%, respectively; p = 0.01), whereas women aged 20–34 years old had a higher decrease of smoking prevalence compared to those ≥35 years (−30.1% vs. −26.5%; p = 0.08), although differences were not statistically significant.
Figure 2 shows time series for LBW, SGA and preterm rate stratified by maternal age categories. LBW and preterm birth rate before and after SBL implementation was highest among women ≥35 years old (7.8% and 10.3%, respectively; p < 0.0001 for comparison with other age categories) and lowest among those between 20 and 34 years old (5.8% and 7.5%, respectively). Prevalence of SGA was highest among women aged <20 years old and lowest among those with 20–34 years (11.7% vs. 9.2%, respectively; p < 0.0001).
FIGURE 2
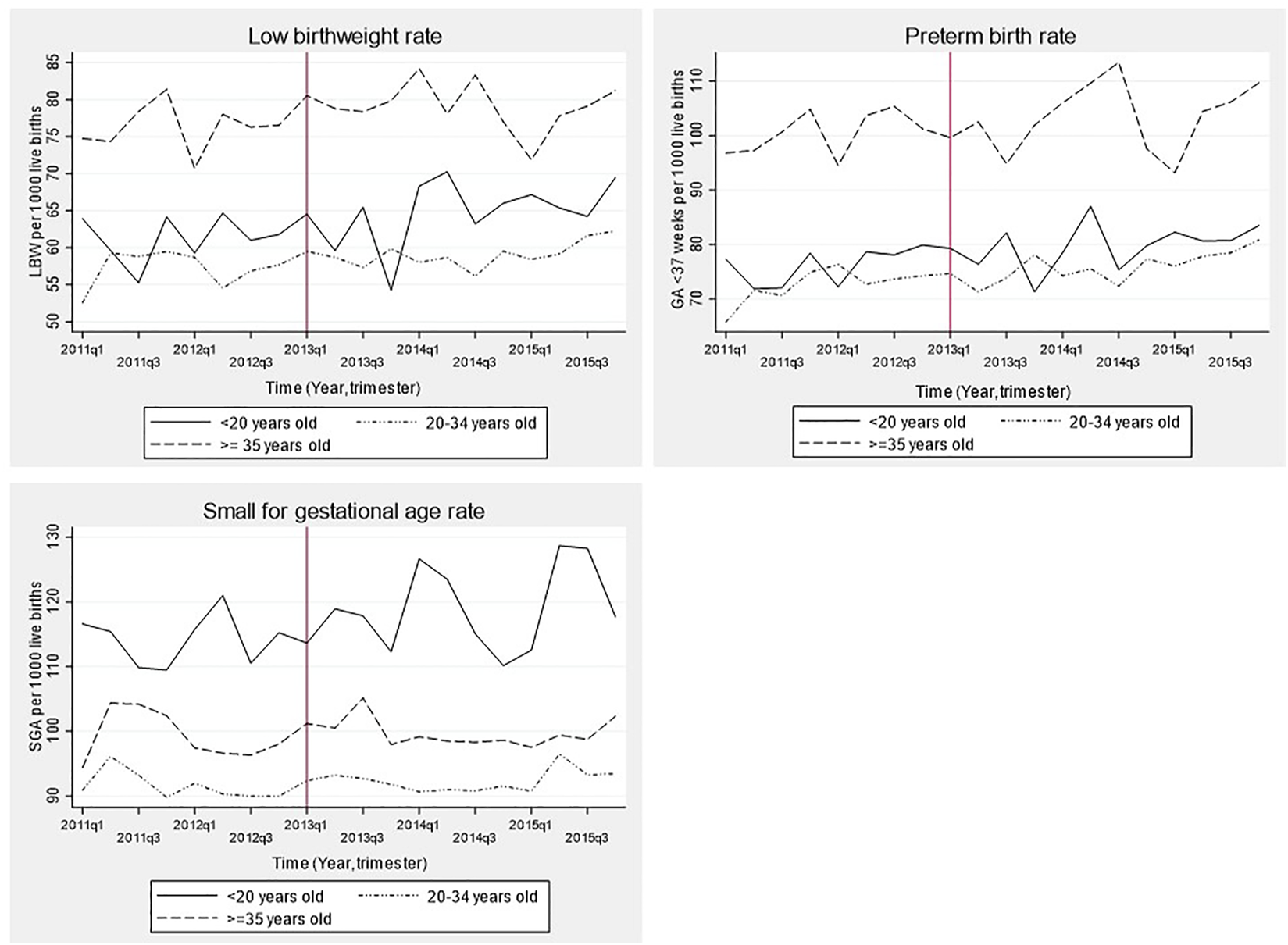
Time series of birth outcomes before and after the implementation of the smoking ban law in Chile stratified by maternal age group, 2011–2015. LBW: Low birth weight (birthweight <2,500 g), GA: Gestational age, SGA: Small for gestational age. Time was a discrete variable measured as 20 trimesters or quarters: from the first trimester of 2011 (time = 0 = 2011q1) to the last trimester of 2015 (time = 19 = 2015q4). The red line shows the implementation of the smoking ban law (trimester including March 2013 = 2013q1).
Figure 3 shows trends in LBW rate before and after SBL implementation for the whole sample (21 cities) and stratified by city density tertiles with their respective incidence rate ratios (IRR) and 95% confidence interval. No significant changes were observed in LBW rate immediately after the intervention (IRR = 0.99 [0.93–1.04]; p = 0.66). Similar results were observed when stratifying by city density tertiles (Figure 3), by maternal age (Figure 4) or when adjusting the models by seasonality (Supplementary Table S1).
FIGURE 3
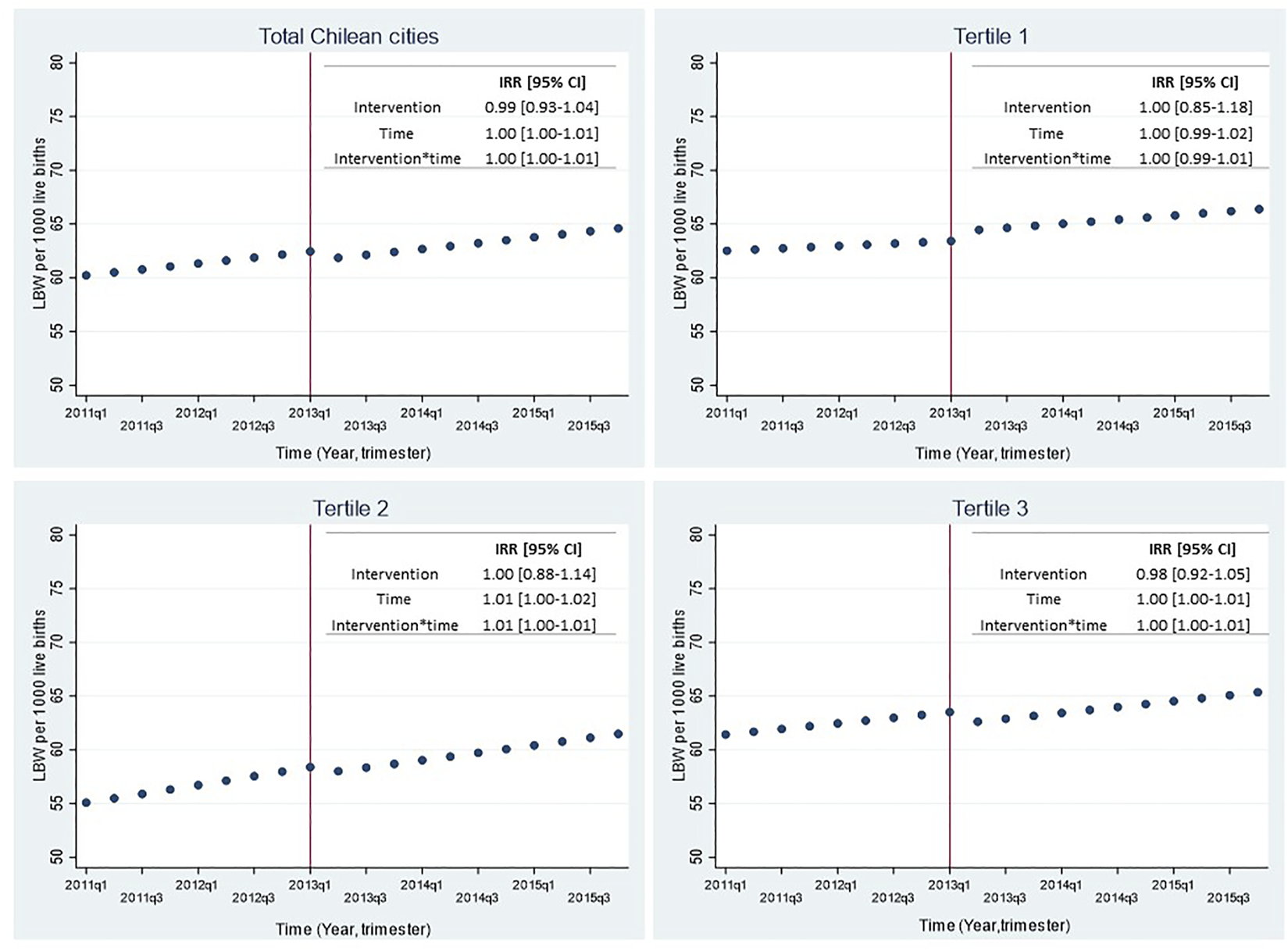
Effect of smoking ban law implementation over low birthweight rate in all 21 Chilean cities and by tertiles of city density, 2011–2015. LBW: Low birth weight (birthweight <2,500 g); IRR: Incidence Rate Ratio; 95% CI: 95% Confidence Interval. The top left figure shows the predicted LBW rate for all 21 Chilean cities; the top right figure shows the predicted LBW rate for cities of the lowest tertile of population density (≤6,153 pers/km2); the bottom left figure shows the predicted LBW rate for cities of the intermediate tertile of population density (6,198–8,132 persons/km2); the bottom right figure shows the predicted LBW rate for cities of the highest tertile of population density (≥8,134 persons/km2). Time was a discrete variable measured as 20 trimesters or quarters: from the first trimester of 2011 (time = 0 = 2011q1) to the last trimester of 2015 (time = 19 = 2015q4). The red line shows the implementation of the smoking ban law (trimester including March 2013 = 2013q1).
FIGURE 4
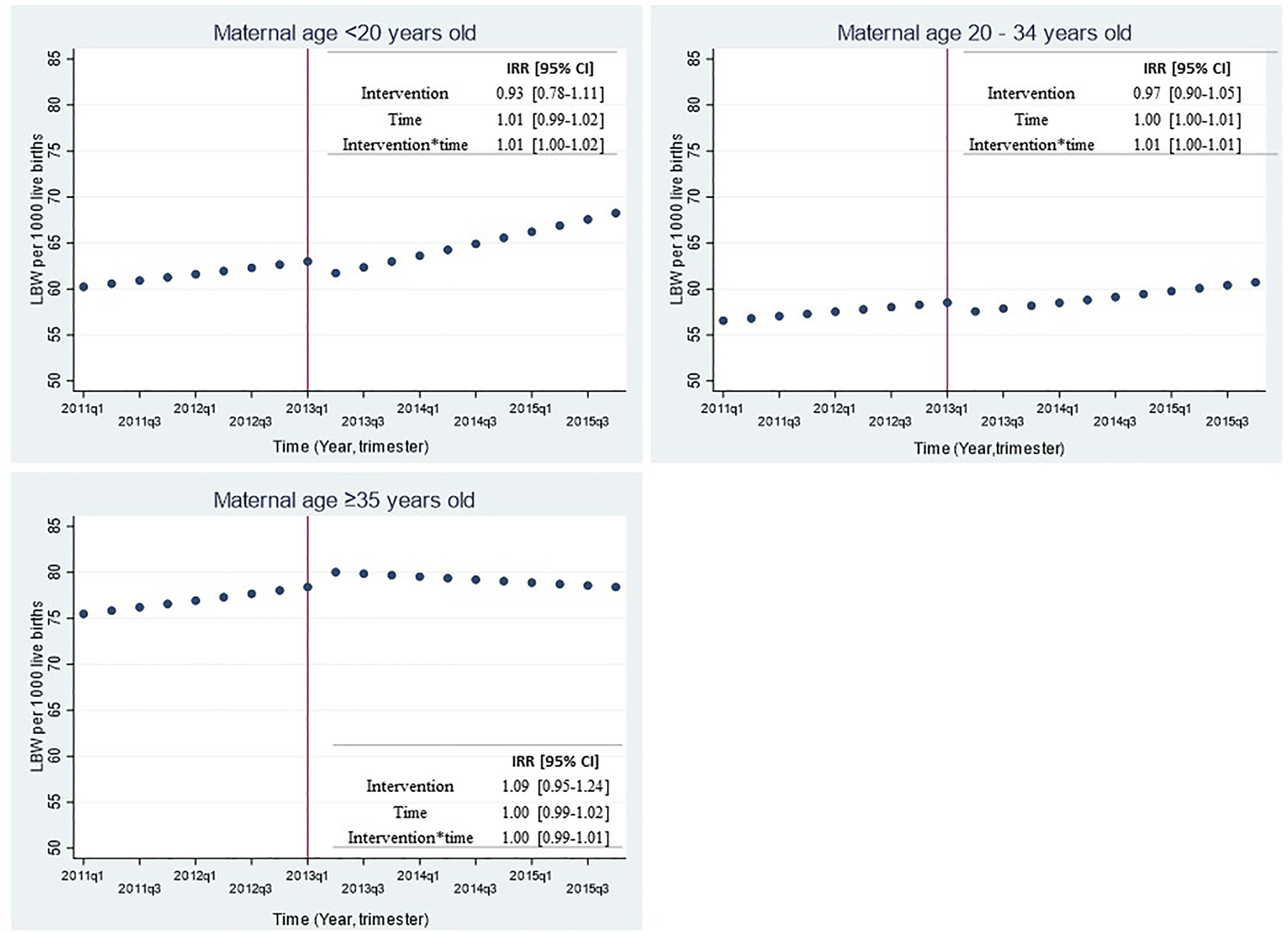
Effect of smoking ban law implementation over low birthweight rate in all 21 Chilean cities by maternal age group 2011-2015. LBW: Low birth weight (birthweight <2500 grams); IRR: Incidence Rate Ratio; 95% CI: 95% Confidence Interval. The top left figure shows the predicted LBW rate for mothers younger than 20 years old; the top right figure shows the predicted LBW rate for mothers between 20 and 34 years old; the bottom left figure shows the predicted LBW rate for mothers with 35 years or older. Time was a discrete variable measured as 20 quarters: from the first quarter or trimester of 2011 (time=0=2011q1) to the last quarter or trimester of 2015 (time=19=2015q4). The red line shows the implementation of the smoking ban law (March 2013).
In the sensitivity analysis, similar results were observed when using months as time-unit (IRR = 0.99 [0.91–1.08]; p = 0.87) and when accounting for lag effects of 3, 6, and 9 months (IRR = 0.98 [0.92–1.04], p = 0.50; IRR = 0.98 [0.90–1.05], p = 0.53; IRR = 0.96 [0.88–1.06], p = 0.44, respectively; Supplementary Figure S2). Sensitivity analysis using LBW rates made of small for gestational age (SGA) as the outcome, did not showed significant changes after the SBL implementation (IRR = 0.98 [0.94–1.02], p = 0.49); similar null results were found when using linear mixed effect models with LBW rate as a continuous outcome (Supplementary Table S1).
Discussion
This study examined changes in smoking and LBW prevalence previous and after the implementation of SBL in Chile and assessed whether these changes varied by city population density and maternal age. Although no significant changes in LBW prevalence have been found immediately after the implementation of SBL in the overall sample of cities and by city density and maternal age, we described significant lower smoking prevalence after SBL implementation in the overall population and across all tertiles of city density, particularly among those in mid-density cities and among women aged 20–34 years old after the implementation of SBL.
We have found no meaningful change in LBW rate after the implementation of SBL. Results from regional or country-level studies using a similar approach are mixed. On one hand, a German study assessing the impact of SBL on perinatal outcomes on Bavaria, showed no significant immediate effect on most of the perinatal outcomes under study (LBW, preterm birth, SGA, and still birth) except from extreme pre-term births [25]. On the other hand, a study in Quebec examining the immediate effects of the SBL and after 3,6, and 9 months on perinatal outcomes showed a significant reduction in SGA, preterm birth and LBW rate only when considering a 9-month lag period [26]. They identified several factors that may explain the heterogeneous findings across studies assessing the effect of smoke-free legislation on pregnancy outcomes, namely: 1) different policy environments in terms of smoking prevalence and smoking norms, 2) the presence of existing legislation prior to the smoke-free legislation under investigation, and 3) differences in policy implementation and enforcement [26]. Peelen et al., who investigated whether immediate changes in perinatal outcomes occurred following SBL and the introduction of key tobacco control policies (taxes and mass media campaign) in the Netherlands reported a reduction in SGA births, but not in LBW [27]. Unlike to those results, we did not find an effect of SBL when considering 3, 6, 9 months- lag periods or SGA rate as an outcome on the sensitivity analysis.
In the case of Chile, the null results of our study could be explained in part by the fact Chile had a long trajectory of public health promotion and programs to reduce tobacco consumption and exposure during critical physiological states such as pregnancy and childhood. These strategies might increase health-conscious behavior among pregnant women, who, prior to the implementation of the smoke-free legislation, already avoided smoke and exposure to secondhand smoke.
Additionally, LBW rates in Chile are low. In 2015, national LBW rate was 6.2% (below the world rate of 14.6%) [28]. We observed lowest rates in mid-density cities (population density between 6198 & 8132 persons/km2 Tertile 2), and, among mothers aged 20–34 years. Chile has implemented interventions to improve perinatal and infant health prior to anti-smoking legislation, such as the creation of the “Chile Crece Contigo” program, which guarantees prenatal care, care for children during the first years of life, improvements in hospital infrastructure, and the increase of institutionalized deliveries, which currently correspond to 99% [29, 30].
In addition to the main results, we found that overall smoking prevalence decreased significantly by 19% after SBL implementation in all cities, which was steeper among younger women (20–34 years old) as has been previously reported in other studies. The relative reductions that we observed in smoking prevalence among young women (−26.5% to −31.1% in women aged 20–49 years) were greater than those recently projected by Flor et al. in the best tobacco restriction scenario (projected -12% to −15.9% in women aged 15–49 years in 175 countries), which combines the smoke-free policy with higher prices, health warnings and banned advertising [31]. Our results showed a greater change in smoking prevalence after SBL implementation in cities with higher population density (≥6,153 person/km2). As bigger and denser cities may present more venues for social interaction and smoking (bars, pubs, restaurants), the impact of SBL implementation in these cities could have been more extensive. Bigger/denser cities could also have more resources to enforce the SBL (more inspections, sanctions, etc.), making the SBL more impactful on health outcomes [19, 31–33]. Even though the anti-smoking law has managed to reduce the smoking prevalence in Chile, recent evidence reports low compliance in night entertainment and semi-open venues in Chile [34, 35]. The SBL has legislative gaps with no progress in a stricter proposal to tobacco restriction aligned with framework agreement of the World Health Organization, which is being discussed by the National Congress [36, 37].
We found a slight increase in the LBW rates over time at the expense of relative higher rates of late- preterm births (34–36 GW: 5.6% and 5.8% before and after SBL, respectively; data in Supplementary Table S2), which are at the higher limit compared to high income countries (3–6%) [38, 39]. This could be linked to an increase in the C-section procedures as common practice for delivery among Chilean women [40, 41]. Overall, Chile is the third country with higher cesarian section rates (∼46%) compared with the OECD countries (27.9%) [38], which is an urban phenomenon as bigger cities count with better hospital infrastructure and technical capacity. De Elejalde & Giolito reported that a reduction in delivery costs in private hospitals for women with public insurance during the last decades in Chile increased C-section probability with a negative effect on birthweight [41].
We accounted for limitations. First, the study design only allows examining short-term impacts of the SBL implementation on LBW rates. Although the null results from this study, it is important to further explore whether SBL enables conditions (such as reducing smoking prevalence among women of childbearing age) that could contribute to a reduction in the LBW rates in the long run. Further studies incorporating more detailed information on smoking prevalence and LBW rates over time could help to better understand this pathway. In addition, we suggest that future studies consider also SGA as a primary outcome of SBL implementation. Second, we were not able to account for other environmental factors influencing birthweight (such as air pollution, multidimensional indicators of poverty, or healthcare access), and other characteristics of cities influencing the enforcement of the SBL (such as density of venues where the SBL was enforced, and penalties associated with infractions to the SBL) that could help to account for between city variability in the association between SBL and LBW rates. However, we assumed that social and built environment variables did not substantially change during the study period (2011–2015) and therefore may not have significant impact in the association under study.
Finally, we were not able to retrieve more individual-level information about maternal characteristics relevant to LBW, such as individual-level smoking behavior, maternal BMI previous to pregnancy, other comorbidities during pregnancy (such as hypertension/diabetes), or previous pregnancies. Additional studies in Chile as well as in other Latin American cities need to consider these limitations when further assessing SBL interventions at the city-level.
Conclusion
SBL implementation did not show significant impact on changes in LBW rate in Chilean cities. However, we were able to describe significant changes in smoking behavior among women of childbearing age, which is a determinant of LBW. Further studies are needed to examine the direct and indirect impact of SBL on perinatal health outcomes.
Statements
Author contributions
GV: Study design, formal analyses, and drafting of the manuscript. AO: Original idea, study design, formal analyses and drafting of the manuscript. LR: Original idea, study design, and drafting of the manuscript. TD: Original idea, study design, and drafting of the manuscript. PM: Formal analyses and editing of the manuscript. CN: Original ideal, study design, and editing of the manuscript.
Funding
This work was supported by the Wellcome Trust initiative “Our Planet, Our Health” (grant 205177/Z/16/Z). The study funder had no role in study design, data collection, data analysis, data interpretation or writing of this study. GV was supported by the Chilean National Agency for Research and Development (ANID) Scholarship Program DOCTORADO NACIONAL under award number 21210712. PHM was supported by the Office of the Director of the National Institutes of Health under award number DP5OD26429 and the Cotswold Postdoctoral Fellowship under award number 284134.
Conflict of interest
The authors declare that they do not have any conflicts of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2022.1605087/full#supplementary-material
References
1.
Blencowe H Krasevec J Onis Mde Black RE An X Stevens GA et al National, Regional, and Worldwide Estimates of Low Birthweight in 2015, with Trends from 2000: a Systematic Analysis. Lancet Glob Health (2019) 7:e849–60. 10.1016/S2214-109X(18)30565-5
2.
Himes SK Stroud LR Scheidweiler KB Niaura RS Huestis MA . Prenatal Tobacco Exposure, Biomarkers for Tobacco in Meconium, and Neonatal Growth Outcomes. J Pediatr (2013) 162(5):970–5. 10.1016/j.jpeds.2012.10.045
3.
Araya C Monge D del Pilar M . REVISTA MEDICINA LEGAL DE COSTA RICA Efectos fetales y posnatales del tabaquismo durante el embarazo Fetal and postnatal effects of smoking during pregnancy. 2019;36(2).
4.
Davies JK Bledsoe JM . Prenatal Alcohol and Drug Exposures in Adoption, 52. Pediatric Clinics of North America Pediatr Clin North Am (2005), 1369–93.
5.
Banderali G Martelli A Landi M Moretti F Betti F Radaelli G et al Short and Long Term Health Effects of Parental Tobacco Smoking during Pregnancy and Lactation: A Descriptive Review, 13. Journal of Translational Medicine BioMed Central Ltd. (2015). p. 327.
6.
Leonardi-Bee J Smyth A Britton J Coleman T . Environmental Tobacco Smoke and Fetal Health: Systematic Review and Meta-Analysis. In: Archives of Disease in Childhood: Fetal and Neonatal Edition Arch Dis Child Fetal Neonatal, 93 (2008).
7.
Castillo-Riquelme M Bardach A Palacios A Pichón-Riviere A . Health burden and Economic Costs of Smoking in Chile: The Potential Impact of Increasing Cigarettes Prices. PLOS ONE. Public Libr Sci (2020) 15(8):e0237967. 10.1371/JOURNAL.PONE.0237967
8.
Encuesta Nacional de Salud. Encuesta Nacional De Salud (2003). [En ligne]. Chile [cité le 27 June 2021]. Disponible: https://www.minsal.cl/portal/url/item/bcb03d7bc28b64dfe040010165012d23.pdf.
9.
Ministerio de Salud de Chile. ENCUESTA NACIONAL DE SALUD 2016-2017. Primeros Resultados. [En ligne] (2017). [cité le 27 October 2021]. Disponible: Available from: https://www.minsal.cl/wp-content/uploads/2017/11/ENS-2016-17_PRIMEROS-RESULTADOS.pdf.
10.
Biblioteca del Congreso Nacional. Ley 19419: Regula actividades que indica relacionadas con el tabaco. [En ligne] (1995). [cité le 7 March 2022]. Disponible: Available from: https://www.bcn.cl/leychile/navegar?idNorma=30786.
11.
[En ligne] Biblioteca del Congreso Nacional de Chile. Historia de La Ley No 20.660. Modifica Ley N° 19.419, en materia de ambientes libres de humo de tabaco (2021). [cité le 27 June 2021]. Disponible: Available from: https://www.bcn.cl/historiadelaley/historia-de-la-ley/vista-expandida/4450/.
12.
Katikireddi SV Der G Roberts C Haw S . Has Childhood Smoking Reduced Following Smoke-free Public Places Legislation? A Segmented Regression Analysis of Cross-Sectional UK School-Based Surveys. Nicotine Tob Res (2016) 18(7):1670–4. 10.1093/NTR/NTW018
13.
White VM Warne CD Spittal MJ Durkin S Purcell K Wakefield MA . What Impact Have Tobacco Control Policies, Cigarette price and Tobacco Control Programme Funding Had on Australian Adolescents’ Smoking? Findings over a 15-year Period. Addiction (Abingdon, England). Addiction (2011) 106(8):1493–502. 10.1111/J.1360-0443.2011.03429.X
14.
Qian X Gu H Wang L Wang X Xuan Z Zheng P et al Changes in Smoking Prevalence after the Enforcement of Smoking Control Regulations in Urban Shanghai, China: Findings from Two Cross-Sectional Surveys. In: Tobacco Induced Diseases. Crete, Greece: The International Society for the Prevention of Tobacco Induced Diseases (2018). p. 16. 10.18332/TID/91095
15.
Friedman AS Wu RJ . Do Local Tobacco-21 Laws Reduce Smoking Among 18 to 20 Year-Olds? Nicotine and Tobacco Research. Nicotine Tob Res (2020) 22(7):1195–201. 10.1093/ntr/ntz123
16.
Chesnokova A French B Weibe D Camenga DR Yun K . Association between Neighborhood-Level Smoking and Individual Smoking Risk: Maternal Smoking Among Latina Women in Pennsylvania. Public Health Rep (2015) 130(6):672–83. 10.1177/003335491513000617
17.
Perlman SE Chernov C Farley SM Greene CM Aldous KM Freeman A et al Exposure to Secondhand Smoke Among Nonsmokers in New York City in the Context of Recent Tobacco Control Policies: Current Status, Changes over the Past Decade, and National Comparisons. In: Nicotine and Tobacco Research, 18. Oxford University Press (2016). p. 2065–74. 10.1093/ntr/ntw13511
18.
Kabir Z Daly S Clarke V Keogan S Clancy L . Smoking Ban and Small-For-Gestational Age Births in Ireland. PLoS One (2013) 8(3):e57441. 10.1371/journal.pone.0057441
19.
Faber T Kumar A Mackenbach JP Millett C Basu S Sheikh A et al Effect of Tobacco Control Policies on Perinatal and Child Health: a Systematic Review and Meta-Analysis. Lancet Public Health (2017) 2(9):e420–37. 10.1016/S2468-2667(17)30144-5
20.
Kusel J Timm B Lockhart I . The Impact of Smoking in the home on the Health Outcomes of Non-smoker Occupants in the UK. In: Tobacco Induced Diseases, 11. Crete, Greece: E.U. European Publishing (2013). p. 3. 10.1186/1617-9625-11-31
21.
[En ligne] Informe sobre el control del tabaco en la Región de las Américas. Report on Tobacco Control in the Region of the Americas (2018). [cité le 27 June 2021]. Disponible: Available from: https://iris.paho.org/handle/10665.2/49237.
22.
Quistberg DA Roux AVD Bilal U Moore K Ortigoza A Rodriguez DA et al Building a Data Platform for Cross-Country Urban Health Studies: the SALURBAL Study. J Urban Health (2019) 96(2):311–37. 10.1007/S11524-018-00326-0
23.
Bernal JL Cummins S Gasparrini A . Interrupted Time Series Regression for the Evaluation of Public Health Interventions: A Tutorial. In: International Journal of Epidemiology, 46. Oxford University Press (2017). p. 348–55. 10.1093/ije/dyw098
24.
Milad MA Novoa PJM Fabres JB Margarita Samamé MM Aspillaga CM Rama de Neonatología D et al Recomendación sobre Curvas de Crecimiento Intrauterino. Revista chilena de pediatría. Sociedad Chilena de Pediatría (2010) 81(3):264–74. 10.4067/S0370-41062010000300011
25.
Polus S Burns J Hoffmann S Mathes T Mansmann U Been Jv et al Interrupted Time Series Study Found Mixed Effects of the Impact of the Bavarian Smoke-free Legislation on Pregnancy Outcomes. Sci Rep (2021) 11:4209. 10.1038/s41598-021-83774-0
26.
McKinnon B Auger N Kaufman JS . The Impact of Smoke-free Legislation on Educational Differences in Birth Outcomes. J Epidemiol Community Health (1978) 69(10):937–43. 10.1136/JECH-2015-205779
27.
Peelen MJ Sheikh A Kok M Hajenius P Zimmermann LJ Kramer BW et al Tobacco Control Policies and Perinatal Health: A National Quasi-Experimental Study OPEN (2016). 10.1038/srep23907
28.
Banco Mundial. Bebés con bajo peso al nacer (% de nacimientos). [En ligne] (2015). [cité le 2 January 2022]. Disponible: Available from: https://datos.bancomundial.org/indicador/SH.STA.BRTW.ZS?end=2015&start=2000&view=map.
29.
Subsecretaria de la niñez. Gobierno de Chile (2022). [En ligne]. Chile Crece Contigo [cité le 2 January 2022]. Disponible: Available from: https://www.crececontigo.gob.cl/temas-y-recomendaciones/gestacion-y-nacimiento/.
30.
Ministerio de Salud. Departamento de Estadisticas e Información de Salud. [En ligne] (2022). [cité le 2 January 2022]. Disponible: Available from: https://deis.minsal.cl/.
31.
Flor LS Reitsma MB Gupta V Ng M Gakidou E . The Effects of Tobacco Control Policies on Global Smoking Prevalence. Nat Med (2021) 27(2):239–43. 10.1038/s41591-020-01210-8
32.
Hone T Szklo AS Filippidis FT Laverty AA Sattamini I Been Jv. et al Smoke-free Legislation and Neonatal and Infant Mortality in Brazil: Longitudinal Quasi-Experimental Study. Tob Control (2020) 29(3):312–9. 10.1136/TOBACCOCONTROL-2019-054923
33.
Vicedo-Cabrera AM Schindler C Radovanovic D Grize L Witassek F Dratva J et al Benefits of Smoking Bans on Preterm and Early-Term Births: a Natural Experimental Design in Switzerland. Tobacco Control. BMJ Publishing Group Ltd (2016) 25(2):e135–41. 10.1136/TOBACCOCONTROL-2015-052739
34.
Peruga A Molina X Delgado I Matute I Olea A Hirmas M et al Compliance with the Smoking Ban in Enclosed, Semiopen and Open Areas of Workplaces and Public Places in Chile. Tob Control (2021) 30(5):570–3. 10.1136/TOBACCOCONTROL-2020-055632
35.
Peruga A Fu M Molina X Fernández E . Night Entertainment Venues Comply Poorly with the Smoke-free Law in Chile. Gac Sanit (2021) 35(4):402–4. 10.1016/J.GACETA.2020.04.016
36.
Girardi G Rossi F Ruiz-Esquide M Camara de Diputadas y . Proyecto de Ley: Adecua la legislación nacional al estándar del Convenio Marco de la Organización Mundial de Salud para el Control del Tabaco. [En ligne] (2013). [cité le 2 January 2022]. Disponible: Available from: https://www.camara.cl/legislacion/ProyectosDeLey/informes.aspx?prmID=9292&prmBOLETIN=8886-11%20https://www.who.int/fctc/text_download/es/.
37.
Organización Mundial de la Salud. CONVENIO MARCO DE LA OMS PARA EL CONTROL DEL TABACO (2003). cité le 2 January 2022]; Disponible: Available from: https://www.who.int/fctc/text_download/es/.
38.
Mariani GL Vain NE . The Rising Incidence and Impact of Non-medically Indicated Pre-labour Cesarean Section in Latin America. Semin Fetal Neonatal Med (2019) 24(1):11–7. 10.1016/J.SINY.2018.09.002
39.
Delnord M Zeitlin J . Epidemiology of Late Preterm and Early Term Births - an International Perspective. Semin Fetal Neonatal Med (2019) 24(1):3–10. 10.1016/J.SINY.2018.09.001
40.
Sutherland P . Global Health Now, John Hopkins Bloomberg School of Public Health[En ligne]. 2021. Doctors Push C-Sections in Chile, but Women Are Pushing Back | Global Health NOW [cité le 5 January 2022]. Disponible: Available from: https://www.globalhealthnow.org/2021-12/doctors-push-c-sections-chile-women-are-pushing-back.
41.
de Elejalde R Giolito E . More Hospital Choices, More C-Sections: Evidence from Chile. Bonn, Germany: IZA Institute of Labor Economics (2019).
Summary
Keywords
tobacco, maternal & child health, Latin America, smoke-free policy, low birth weight, Chile
Citation
Valentino G, Ortigoza A, Rodriguez Osiac L, Doberti T, Mullachery P and Nazzal C (2022) Smoking Ban Law in Chile: Impact in Newborns’ Birth Weight by Women’s Age Groups and by City Population Density. Int J Public Health 67:1605087. doi: 10.3389/ijph.2022.1605087
Received
26 May 2022
Accepted
25 November 2022
Published
12 December 2022
Volume
67 - 2022
Edited by
Nino Kuenzli, Swiss Tropical and Public Health Institute (Swiss TPH), Switzerland
Updates
Copyright
© 2022 Valentino, Ortigoza, Rodriguez Osiac, Doberti, Mullachery and Nazzal.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Ortigoza, afo25@drexel.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.