Abstract
Objectives: To examine associations between maternal sulfur dioxide (SO2) exposure and congenital ear malformations risk in offspring.
Methods: We surveyed 1676 cases with congenital ear malformations and 7950 controls from the Maternal and Child Health Certificate Registry of Liaoning Province between 2010 and 2015. SO2 concentrations were obtained from the Municipal Environment Protection Bureau of Liaoning Province. Multivariable logistic regression models and Restricted cubic splines (RCS) model were used to assess the aforementioned association.
Results: There were significant associations between maternal SO2 exposure and congenital ear malformations risk during the 3 months before conception (OR Q4 vs. Q1 = 1.93, 95% CI = 1.43–2.59) and the 3 months after conception (OR Q4 vs. Q1 = 1.63, 95% CI = 1.22–2.18). Similar results were obtained in the analysis of single-month exposure windows, except for the third month before conception and the third month after conception. Moreover, these findings were broadly consistent across subgroups and robust in sensitivity analyses. There were non-linear dose-response associations between SO2 exposure and congenital ear malformations based on restricted cubic spline model analysis.
Conclusion: Maternal SO2 exposure is associated with increased congenital ear malformations risk in offspring.
Introduction
Congenital ear malformations are defined as deformities caused by congenital embryonic developmental disorders. Congenital ear malformations are comprised of microtia, including anotia, and other malformations of the external ear that exclude microtia and anotia. Among these, the main type of congenital ear malformations is microtia [1].
Globally, the prevalence of microtia varies greatly, ranging from 0.83/10,000 to 17.4/10,000 [2]. Based on the China Maternal and Child Health Monitoring and Annual Report, the incidence of microtia in China in 2018 was 2.99 per 10,000 cases [3]. The incidence of congenital ear malformations remains high, which creates great physiological and psychological obstacles and economic burdens to patients. However, no clear cause and mechanism have been found for this disease. Moreover, ear malformations are a multifactorial disease, which may be caused by environmental and genetic factors, as well as interactions between these factors [4, 5]. It has been reported that more than 2 million premature deaths each year are attributed to air pollution, and 91% of the world’s population live in environments where air quality does not meet the standards set by the World Health Organization [6].
In 2002, Ritz et al. [7] first discovered the association between ambient air pollution and congenital malformations, which has since been confirmed by numerous researchers [8–13]. Although evidence from in vivo and in vitro studies [14–18] seem to indicate that air pollution exposure during pregnancy causes congenital ear malformations, evidence from epidemiological studies remain limited.
Liaoning Province is one of the important old industrial bases in China. Presently, the province has 39 major industries, 197 medium industries, and more than 500 small industries, making it one of the provinces with the most complete industrial profile in China. The rapid development of industries greatly affects the atmospheric environment. The 2016 China Environmental Bulletin, which has been issued by the Ministry of Ecology and Environment of the People’s Republic of China, stated that among China’s 338 prefecture-level and higher cities, 254 of them exceed environmental air quality standards, accounting for 75.1% of cities (http://www.mee.gov.cn), and the sulfur dioxide (SO2) concentration ranged from 3 to 88 μm/m3, with an average of 22 μm/m3. The average annual SO2 concentration is 34 μm/m3 in Liaoning Province, which is much higher than the national average. In 2014, the annual population-weighted-average value of SO2 in China was 34.1 μm/m3, and it was 96.7% higher in northern China than that in southern China [19]. Thus, our study seeks to expand the etiological research of congenital ear malformations. The purpose of this case-control study is to estimate the association between maternal SO2 exposure and congenital ear malformations risk in offspring during a crucial period.
Methods
Study Population and Data Source
The study population was comprised of offspring with congenital ear malformations and healthy infants from 1 January 2010 to 31 December 2015. The study population was recruited from the Maternal and Child Health Certificate Registry of Liaoning Province, which is managed by Liaoning Women and Children’s Health Hospital. Details on the registry have been previously published [20, 21]. In brief, the registry is an active hospital-based monitoring system that is maintained in accordance with the national monitoring program. All 31 provinces in China have launched the active monitoring system. Similar to other provinces, Liaoning Province submits data to a national database maintained by the China Birth Defects Monitoring Network [22]. All 14 major cities in Liaoning Province (Shenyang, Dalian, Anshan, Fushun, Benxi, Dandong, Jinzhou, Yingkou, Fuxin, Liaoyang, Panjin, Tieling, Chaoyang, and Huludao) were covered by the Maternal and Child Health Certificate Registry. Data on congenital malformations were provided by Maternal and Child Health Certificate Registries in 14 cities of Liaoning Province and collected from Liaoning Women and Children’s Health Hospital [22]. During the study period from 2010 to 2015, approximately 6,000 cases of congenital malformations were reported each year from all maternity units in the province [23].
Cases and Controls
Cases with congenital ear malformations (n = 1676) were registered in the Maternal and Child Health Certificate Registry of Liaoning Province and classified as live births, stillbirths, or pregnancy terminations after prenatal diagnosis of congenital anomalies that were born or terminated from 2010 to 2015. According to the International Classification of Diseases, 10th Revision, Clinical Modifications [ICD-10-CM], congenital ear malformations include two major malformations: microtia (including anotia; n = 361; ICD10:Q17.2, Q16.0) and other external ear malformations (except for microtia and anotia; n = 1315; ICD10:Q17).
Controls were selected as previously described [20]. In brief, we divided Liaoning Province into southeastern, western, and central regions according to geographical features. Thereafter, five cities from the three regions (southeastern region: Dalian; western region: Fuxin, Chaoyang, and Huludao; central region: Shenyang) were representatively selected based on the degree of air pollution and socio-economic characteristics. The control group was a random sampling of 1.5% of live births without congenital ear malformations from the five cities in the three regions by random birth-year sampling, which was not case-related (mismatched). Subjects with missing or implausible covariate information were excluded and not included in the final analysis. We conducted this study according to national and local regulations. The Institutional Review Board of Liaoning Women and Children’s Health Hospital reviewed and approved the study protocol, and the study was conducted in compliance with local and national regulations.
Data Collection and Quality Control
The data collection process was carried out as previously described [21]. In brief, each newborn or terminated fetus was inspected by an experienced obstetric or pediatric specialist after birth. Cases were registered and coded based on the International Classification of Diseases, 10th Edition (ICD10). For cases of suspected congenital ear malformations that were diagnosed by prenatal ultrasonography, we examined newborns or fetuses again after postpartum check or pregnancy termination. Cases were diagnosed in all monitored hospitals from the 14 cities in Liaoning Province, and experienced obstetric or pediatric specialists immediately interviewed the newborn’s mother in order to complete the Birth Defects Registration Form that was used to gather data, including information on maternal and child demographics, diagnosis of congenital defects, history of early pregnancy, and family history. Forms were submitted to the local Maternal and Child Healthcare Institution in the 14 cities, which submitted forms to the Liaoning Women and Children’s Health Hospital. Data were retrospectively verified by a team of experienced genetics and pediatrics specialists [24].
The data quality control was carried out as previously described [24]. Based on the Chinese Maternal and Child Health Surveillance Workbook, specialists from monitoring institutions at all levels diagnosed the disease, and collected and checked the data, as well as the medical records to ensure the high quality of data. To distinguish between inadequacies and inaccuracies, the experts also carried out an independent retrospective investigation [24].
Exposure Assessment
The environmental air pollutant monitoring station network in Liaoning Province is comprised of 77 monitoring stations in 14 cities (two of which served as controls), and this network assessed air pollutant exposure (except for the two controls) (Figure 1). The 77 air pollutant monitoring stations in Liaoning Province were mainly located in urban areas, where they covered residential areas and represented air pollutant levels in the region. According to the Chinese government’s ambient air quality standards from the China National Environmental Monitoring Centre (CNEMC, http://www.cnemc.cn), all monitoring stations in the 14 cities detected and reported air pollutant concentrations and submitted these data to the Environment Protection Bureau. The exposure levels were assessed using the mean SO2 concentration at each monitoring station in the mother’s city of residence, and then these values were used to calculate the monthly mean SO2 concentration (Supplementary Figure S1). Monthly mean SO2 concentration exposure levels were assigned to each newborn after averaging concentrations from all air pollutant monitoring stations recorded by the Birth Defects Registration Form during the 3 months before conception and the 3 months after conception. The gestational age of cases and controls was estimated according to the due date provided by the obstetrician. We used the date of the last menstrual period to determine the first month of pregnancy. If the date of the last menstrual period occurred in the first half of the month, then this month was considered as the first month of conception. Otherwise, it was considered as the first month before conception.
FIGURE 1
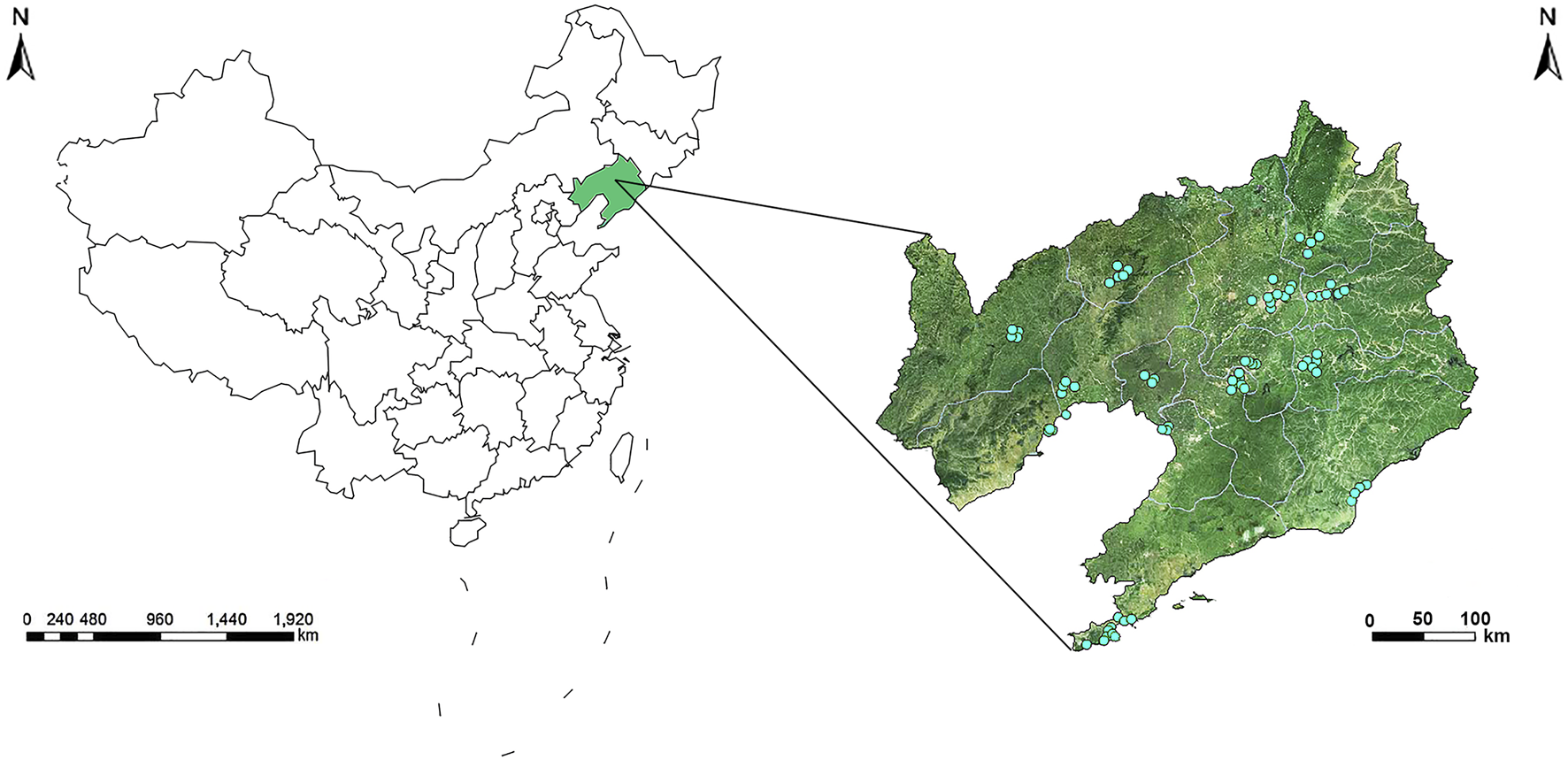
Geographic locations of air monitoring stations in 14 cities in Liaoning Province, China (Association between maternal exposure to SO2 and congenital ear malformations in offspring: a population-based case-control study in Liaoning Province, China, 2010–2015).
Covariates
According to the change in the estimate criterion (10%) [25], we selectively adjusted the corresponding covariates in the model. In the final model, the covariates were maternal age (two categories: <30, ≥30 years), season of conception (four categories: spring [March–May], summer [June–August], fall [September–November], and winter [December–February]), gravidity (two categories: 1, ≥2), parity (two categories: 1, ≥2), maternal education (four categories: elementary school or less, middle school, high school, and college or above), and maternal NO2 and PM10 exposure levels.
Statistical Analysis
Congenital ear malformations were treated as a dichotomous category and analyzed as a dependent variable. From the third month before conception to the third month after conception, the SO2 exposure concentration was computed as a continuous independent variable and a categorical independent variable based on quartiles of distribution in controls. Characteristics of the categorical variables in cases and controls were presented as frequencies and percentages, and they were compared using the Chi-square test. Means, standard deviations (SD), medians, and percentiles were used to characterize the monthly mean ambient SO2 concentration in the 14 cities in Liaoning Province. Spearman’s rank correlation was applied to analyze the correlation between three pollutants (SO2, NO2, and PM10). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated by multivariable logistic regression analysis. We also examined the associations between maternal SO2 exposure, congenital ear malformations, and subgroups in offspring. Specifically, SO2 exposure concentrations were divided into quartiles (determined from controls), and the other quartiles (second, third, and fourth) were compared to a reference (the first quartile) to calculate ORs. We conducted linear tests using the median values of SO2 exposure in each category as a continuous variable and congenital ear malformations as the response variable. Additionally, ORs in both per 1-standard deviation (SD) and per 10 μm/m3 increment were also reported. In our analysis, we respectively fitted single-pollutant (SO2 and the aforementioned covariates) and multi-pollutant models (SO2+NO2; SO2+PM10; SO2+NO2+PM10) to assess the effects of different pollutants. Furthermore, subgroup analyses were used to investigate the associations between SO2 exposure, congenital ear malformations, and maternal age (<30, ≥30 years) using stratification. We added a multiplicative interaction in the regression model between exposure and age to measure the potential interactions.
We performed a sensitivity analysis using propensity score matching (PSM) analysis, which can reduce baseline group differences. Cases with congenital ear malformations (n = 1676) and controls (n = 7950) were matched using 1:1 nearest-neighbor matching with a caliper width equal to 0.2 of SD of the logit of propensity scores. And propensity scores were estimated by multivariable logistic regression model, with adjusting for maternal age, season of conception, gravidity, parity, and maternal education. If a case subject could not be matched to any control subject, then the case subject was excluded. Absolute standardized differences (ASD) were used to confirm the balance of covariates between cases and matched control individuals [26]. An ASD of less than 10% was considered a negligible difference between cases and controls.
For associations in the multivariable logistic regression analysis, we further used a restricted cubic splines (RCS) model to assess their shapes of dose-response association [27]. Nonlinear associations were modeled by penalized cubic splines with 3 equally spaced knots. For the first and the second months after conception, the minimum value was defined as the referent value by default in the SAS macro “%RCS_Reg” [27]. The 5th percentile value was defined as the reference in the other six exposure windows.
All statistical analyses were conducted using SAS software version 9.4 (SAS Institute Inc., Cary, North Carolina, United States). Statistical significance was taken at p-values < 0.05 based on two-sided tests.
Results
The basic characteristics of cases and controls in Liaoning Province are shown in Table 1. Cases of congenital ear malformations (n = 1676) and controls (n = 7950) were included in our analysis. Compared with controls, the number of males was greater (57.0%), the gestational age was shorter (<37 weeks, 6.1%), the birth weight was lower (<2500 g, 5.3%), and more births occurred within the fall (26.3%) and the winter (20.8%) for cases. Compared with controls, the mothers of cases were younger (<30 years, 64.4%), less educated (high school or less, 66.9%), and had more pregnancies (≥2, 45.2%) and deliveries (≥2, 19.6%).
TABLE 1
| Characteristics | Cases | Controls | p-Value |
|---|---|---|---|
| Total | 1676 (100) | 7950 (100) | |
| Season of conception | <0.001 | ||
| Spring | 443 (26.4) | 2106 (26.5) | |
| Summer | 444 (26.5) | 2829 (35.6) | |
| Fall | 440 (26.3) | 1705 (21.4) | |
| Winter | 349 (20.8) | 1310 (16.5) | |
| Gender of infant | <0.001 | ||
| Female | 720 (43.0) | 3927 (49.4) | |
| Male | 956 (57.0) | 4023 (50.6) | |
| Gestational age, weeks | <0.001 | ||
| <37 | 102 (6.1) | 257 (3.2) | |
| ≥37 | 1574 (93.9) | 7693 (96.8) | |
| Birth weight, grams | <0.001 | ||
| <2500 | 89 (5.3) | 174 (2.2) | |
| 2500–<4000 | 1410 (84.1) | 6840 (86.0) | |
| ≥4000 | 177 (10.6) | 936 (11.8) | |
| Maternal age, years | <0.001 | ||
| <30 | 1080 (64.4) | 4704 (59.2) | |
| ≥30 | 596 (35.6) | 3246 (40.8) | |
| Gravidity | <0.001 | ||
| 1 | 918 (54.8) | 5026 (63.2) | |
| ≥2 | 758 (45.2) | 2924 (36.8) | |
| Parity | <0.001 | ||
| 1 | 1348 (80.4) | 7695 (96.8) | |
| ≥2 | 328 (19.6) | 255 (3.2) | |
| Maternal education | <0.001 | ||
| Elementary school or less | 68 (4.1) | 265 (3.3) | |
| Middle school | 659 (39.3) | 2912 (36.6) | |
| High school | 394 (23.5) | 1723 (21.7) | |
| College or above | 555 (33.1) | 3050 (38.4) |
Characteristics of controls and cases in Liaoning Province, China, 2010–2015 [no.(%)] (Association between maternal exposure to SO2 and congenital ear malformations in offspring: a population-based case-control study in Liaoning Province, China, 2010–2015).
Table 2 shows the distribution characteristics of the ambient SO2 concentration (μg/m³) in the 14 cities in Liaoning Province from 2010 to 2015. The results of our analysis showed that exposure to the mean SO2 concentration in cases was slightly equal to that in controls, whereas exposure to the mean NO2 concentration and PM10 exposure in cases was slightly lower than that in controls during the 3 months before and the 3 months after conception (Table 3). Meanwhile, there were high correlations of SO2 and PM10 during the 3 months before conception (r = 0.71) and the 3 months after conception (r = 0.78).
TABLE 2
| Characteristics | Mean ± SD | Range | Median (25tile-75tile) |
|---|---|---|---|
| Years | |||
| 2010 | 42 ± 27 | 136 | 33 (23–54) |
| 2011 | 42 ± 31 | 139 | 30 (19–60) |
| 2012 | 39 ± 31 | 188 | 29 (16–55) |
| 2013 | 43 ± 37 | 252 | 30 (19–58) |
| 2014 | 46 ± 35 | 191 | 34 (21–64) |
| 2015 | 40 ± 33 | 191 | 28 (15–58) |
| Cities | |||
| Shenyang | 69 ± 59 | 246 | 41 (27–102) |
| Dalian | 34 ± 28 | 108 | 22 (11–59) |
| Anshan | 51 ± 43 | 154 | 28 (17–86) |
| Fushun | 38 ± 22 | 83 | 31 (21–51) |
| Benxi | 51 ± 38 | 133 | 41 (19–78) |
| Dandong | 36 ± 28 | 92 | 20 (15–61) |
| Jinzhou | 42 ± 29 | 109 | 31 (22–55) |
| Yingkou | 31 ± 22 | 78 | 22 (14–47) |
| Fuxin | 47 ± 22 | 91 | 41 (30–60) |
| Liaoyang | 47 ± 27 | 123 | 38 (28–61) |
| Panjin | 25 ± 12 | 55 | 22 (17–29) |
| Tieling | 31 ± 20 | 84 | 25 (15–42) |
| Chaoyang | 39 ± 26 | 99 | 29 (18–58) |
| Huludao | 46 ± 28 | 103 | 35 (24–65) |
Ambient SO2 concentrations (μg/m3) in 14 cities in Liaoning province, China, 2010-2015 (Association between maternal exposure to SO2 and congenital ear malformations in offspring: a population-based case-control study in Liaoning Province, China, 2010–2015).
Abbreviations: SD, standard deviation; tile, percentile.
TABLE 3
| Air pollutant (μg/m3) | Exposure window | Cases (n = 1676) | Controls (n = 7950) | Correlation coefficients | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (25tile-75tile) | Mean ± SD | Median (25tile-75tile) | SO2 | NO2 | PM10 | ||
| SO2 | The 3 months before conception | 47 ± 38 | 35 (22–63) | 48 ± 41 | 34 (23–59) | 1 | 0.50 | 0.71 |
| NO2 | 34 ± 10 | 34 (28–41) | 37 ± 9 | 36 (31–42) | 1 | 0.50 | ||
| PM10 | 88 ± 24 | 86 (71–101) | 93 ± 27 | 89 (75–105) | 1 | |||
| SO2 | The 3 months after conception | 47 ± 36 | 35 (23–63) | 47 ± 42 | 30 (21–65) | 1 | 0.56 | 0.78 |
| NO2 | 35 ± 10 | 34 (28–41) | 37 ± 9 | 35 (31–42) | 1 | 0.53 | ||
| PM10 | 88 ± 25 | 86 (70–101) | 91 ± 28 | 87 (68–106) | 1 | |||
The distribution and correlation of air pollutants’ mean level during the 3 months before conception and the 3 months after conception (Association between maternal exposure to SO2 and congenital ear malformations in offspring: a population-based case-control study in Liaoning Province, China, 2010–2015).
Abbreviations: SD, standard deviation; tile, percentile.
There was a significant association between maternal SO2 exposure and congenital ear malformations risk during the 3 months before conception and the 3 months after conception. The deleterious effects of maternal SO2 exposure on congenital ear malformations remained strong during the exposure window of each single month, except for the third month before conception and the third month after conception (Table 4). When investigating congenital ear malformations subtypes, the higher effect estimates of SO2 were observed among conception in external ear malformations compared to in microtia during almost the entire exposure window (Supplementary Table S1). The risk of congenital ear malformations to SO2 exposure during the 3 months before conception and the 3 months after conception was greater for younger mothers (age <30 years old) (Supplementary Table S2).
TABLE 4
| Quartile of SO2 levela | No. of cases | No. of controls | Unadjusted OR (95% CI) | Model 1b (95% CI) | Model 2c (95% CI) | Model 3d (95% CI) | Model 4e (95% CI) |
|---|---|---|---|---|---|---|---|
| Pre-conception, 0–1 month | |||||||
| <19 | 401 | 1983 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 19 to <29 | 337 | 1931 | 0.86 (0.74–1.01) | 0.83 (0.70–0.98) | 0.83 (0.70–0.98) | 0.89 (0.75–1.06) | 0.85 (0.72–1.02) |
| 29 to <52 | 432 | 2048 | 1.04 (0.90–1.21) | 0.87 (0.73–1.05) | 1.02 (0.84–1.23) | 1.07 (0.88–1.29) | 1.09 (0.90–1.33) |
| ≥52 | 506 | 1988 | 1.26 (1.09–1.46) | 0.94 (0.76–1.17) | 1.48 (1.17–1.87) | 1.27 (1.01–1.60) | 1.61 (1.27–2.05) |
| Ptrend | <0.001 | 0.803 | <0.001 | 0.012 | <0.001 | ||
| Per 1-SD increase | 1.03 (0.98–1.09) | 0.83 (0.76–0.91) | 1.10 (1.00–1.20) | 1.06 (0.95–1.18) | 1.24 (1.11–1.38) | ||
| Per 10 μg/m3 increase | 1.01 (1.00–1.02) | 0.96 (0.94–0.98) | 1.02 (1.00–1.04) | 1.01 (0.99–1.04) | 1.05 (1.02–1.07) | ||
| Pre-conception, 1–2 months | |||||||
| <19 | 369 | 1713 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 19 to <31 | 404 | 2225 | 0.84 (0.72–0.98) | 0.84 (0.71–0.99) | 0.88 (0.75–1.05) | 0.91 (0.77–1.08) | 0.92 (0.77–1.09) |
| 31 to <54 | 399 | 1949 | 0.95 (0.81–1.11) | 0.98 (0.81–1.18) | 1.17 (0.96–1.42) | 1.25 (1.03–1.52) | 1.28 (1.05–1.58) |
| ≥54 | 504 | 2063 | 1.13 (0.98–1.32) | 1.02 (0.81–1.28) | 1.63 (1.27–2.08) | 1.43 (1.13–1.83) | 1.83 (1.42–2.38) |
| Ptrend | 0.001 | 0.476 | <0.001 | 0.003 | <0.001 | ||
| Per 1-SD increase | 1.00 (0.95–1.05) | 0.87 (0.80–0.94) | 1.11 (1.02–1.20) | 1.07 (0.97–1.18) | 1.23 (1.11–1.36) | ||
| Per 10 μg/m3 increase | 1.00 (0.99–1.01) | 0.97 (0.95–0.99) | 1.02 (1.00–1.04) | 1.01 (0.99–1.04) | 1.05 (1.02–1.07) | ||
| Pre-conception, 2–3 months | |||||||
| <23 | 511 | 1728 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 23 to <35 | 363 | 2186 | 0.56 (0.48–0.65) | 0.55 (0.47–0.65) | 0.61 (0.51–0.72) | 0.59 (0.50–0.70) | 0.61 (0.52–0.73) |
| 35 to <67 | 388 | 1959 | 0.67 (0.58–0.78) | 0.57 (0.47–0.70) | 0.79 (0.64–0.96) | 0.66 (0.54–0.80) | 0.80 (0.65–0.98) |
| ≥67 | 414 | 2077 | 0.67 (0.58–0.78) | 0.47 (0.37–0.60) | 0.87 (0.68–1.11) | 0.63 (0.49–0.81) | 0.90 (0.69–1.17) |
| Ptrend | 0.005 | <0.001 | 0.586 | 0.050 | 0.351 | ||
| Per 1-SD increase | 0.90 (0.85–0.95) | 0.78 (0.72–0.85) | 1.02 (0.93–1.12) | 0.91 (0.82–1.01) | 1.07 (0.96–1.20) | ||
| Per 10 μg/m3 increase | 0.98 (0.97–0.99) | 0.95 (0.93–0.97) | 1.00 (0.99–1.02) | 0.98 (0.96–1.00) | 1.02 (0.99–1.04) | ||
| Pre-conception, 0–3 months | |||||||
| <23 | 466 | 1900 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 23 to <34 | 343 | 2080 | 0.67 (0.58–0.78) | 0.59 (0.50–0.70) | 0.79 (0.66–0.93) | 0.74 (0.62–0.88) | 0.87 (0.73–1.04) |
| 34 to <59 | 401 | 1981 | 0.83 (0.71–0.96) | 0.71 (0.58–0.87) | 1.03 (0.83–1.28) | 0.98 (0.79–1.21) | 1.21 (0.96–1.51) |
| ≥59 | 466 | 1989 | 0.96 (0.83–1.10) | 0.66 (0.51–0.84) | 1.40 (1.07–1.83) | 1.27 (0.96–1.68) | 1.93 (1.43–2.59) |
| Ptrend | 0.121 | 0.100 | <0.001 | <0.001 | <0.001 | ||
| Per 1-SD increase | 0.98 (0.92–1.03) | 0.81 (0.74–0.88) | 1.07 (0.98–1.17) | 1.13 (1.01–1.26) | 1.36 (1.20–1.53) | ||
| Per 10 μg/m3 increase | 0.99 (0.98–1.01) | 0.95 (0.93–0.97) | 1.02 (1.00–1.04) | 1.03 (1.00–1.06) | 1.08 (1.05–1.11) | ||
| Post-conception, 0–1 month | |||||||
| <17 | 322 | 1896 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 17 to <29 | 431 | 2051 | 1.24 (1.06–1.45) | 1.15 (0.97–1.37) | 1.11 (0.93–1.31) | 1.29 (1.09–1.53) | 1.17 (0.98–1.39) |
| 29 to <52 | 411 | 1958 | 1.24 (1.06–1.45) | 0.95 (0.78–1.15) | 1.11 (0.91–1.35) | 1.26 (1.03–1.55) | 1.23 (1.00–1.52) |
| ≥52 | 512 | 2045 | 1.47 (1.27–1.72) | 0.89 (0.71–1.13) | 1.51 (1.19–1.92) | 1.44 (1.12–1.85) | 1.77 (1.37–2.29) |
| Ptrend | <0.001 | 0.199 | <0.001 | 0.028 | <0.001 | ||
| Per 1-SD increase | 1.01 (0.96–1.07) | 0.76 (0.69–0.83) | 1.02 (0.93–1.12) | 0.96 (0.85–1.08) | 1.13 (1.01–1.27) | ||
| Per 10 μg/m3 increase | 1.00 (0.99–1.01) | 0.94 (0.92–0.96) | 1.00 (0.98–1.03) | 0.99 (0.97–1.02) | 1.03 (1.00–1.05) | ||
| Post-conception, 1–2 months | |||||||
| <17 | 318 | 1960 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 17 to <29 | 427 | 1866 | 1.41 (1.20–1.65) | 1.30 (1.10–1.54) | 1.27 (1.07–1.51) | 1.44 (1.21–1.72) | 1.31 (1.10–1.57) |
| 29 to <58 | 436 | 2087 | 1.29 (1.10–1.51) | 0.96 (0.79–1.17) | 1.24 (1.02–1.51) | 1.22 (1.00–1.49) | 1.32 (1.08–1.62) |
| ≥58 | 495 | 2037 | 1.50 (1.29–1.75) | 0.80 (0.63–1.02) | 1.34 (1.04–1.73) | 1.22 (0.94–1.60) | 1.49 (1.14–1.95) |
| Ptrend | <0.001 | 0.007 | 0.111 | 0.703 | 0.039 | ||
| Per 1-SD increase | 1.00 (0.94–1.05) | 0.75 (0.69–0.82) | 0.99 (0.90–1.09) | 0.89 (0.79–0.99) | 1.03 (0.92–1.16) | ||
| Per 10 μg/m3 increase | 1.00 (0.99–1.01) | 0.94 (0.92–0.96) | 1.00 (0.98–1.02) | 0.97 (0.95–1.00) | 1.01 (0.98–1.03) | ||
| Post-conception, 2–3 months | |||||||
| <18 | 347 | 1932 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 18 to <32 | 433 | 1906 | 1.27 (1.08–1.48) | 1.12 (0.94–1.33) | 1.14 (0.96–1.36) | 1.25 (1.05–1.49) | 1.20 (1.01–1.43) |
| 32 to <66 | 485 | 2105 | 1.28 (1.10–1.49) | 0.90 (0.74–1.09) | 1.17 (0.96–1.44) | 1.15 (0.93–1.41) | 1.28 (1.04–1.59) |
| ≥66 | 411 | 2007 | 1.14 (0.98–1.33) | 0.56 (0.43–0.72) | 1.00 (0.76–1.30) | 0.85 (0.65–1.11) | 1.15 (0.87–1.52) |
| Ptrend | 0.599 | <0.001 | 0.427 | 0.014 | 0.936 | ||
| Per 1-SD increase | 0.96 (0.91–1.01) | 0.70 (0.64–0.77) | 0.93 (0.84–1.02) | 0.84 (0.75–0.94) | 1.00 (0.89–1.13) | ||
| Per 10 μg/m3 increase | 0.99 (0.98–1.00) | 0.93 (0.91–0.95) | 0.98 (0.96–1.01) | 0.96 (0.94–0.99) | 1.00 (0.98–1.03) | ||
| Post-conception, 0–3 months | |||||||
| <21 | 378 | 2002 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 21 to <30 | 345 | 2028 | 0.90 (0.77–1.06) | 0.81 (0.68–0.96) | 0.95 (0.80–1.13) | 1.00 (0.84–1.19) | 1.05 (0.88–1.25) |
| 30 to <65 | 549 | 1892 | 1.54 (1.33–1.78) | 0.98 (0.81–1.19) | 1.37 (1.12–1.68) | 1.45 (1.17–1.79) | 1.64 (1.32–2.03) |
| ≥65 | 404 | 2028 | 1.06 (0.91–1.23) | 0.54 (0.42–0.70) | 1.19 (0.91–1.55) | 1.12 (0.84–1.48) | 1.63 (1.22–2.18) |
| Ptrend | 0.303 | <0.001 | 0.873 | 0.292 | 0.063 | ||
| Per 1-SD increase | 0.99 (0.93–1.04) | 0.70 (0.63–0.76) | 0.96 (0.87–1.06) | 0.98 (0.86–1.11) | 1.18 (1.03–1.34) | ||
| Per 10 μg/m3 increase | 1.00 (0.98–1.01) | 0.92 (0.90–0.94) | 0.99 (0.97–1.01) | 1.00 (0.97–1.03) | 1.04 (1.01–1.07) | ||
Associations between maternal exposure to ambient SO2 during various exposure windows and the risk of congenital ear malformations in offspring (Association between maternal exposure to SO2 and congenital ear malformations in offspring: a population-based case-control study in Liaoning Province, China, 2010–2015).
Abbreviations: CI, confidence interval; OR, odds ratios; SD, standard deviation; SO2, sulfur dioxide; ref, reference.
SO2 concentrations (μg/m³) are based on the monthly average concentrations, which are then averaged over different exposure windows and analyzed in quartiles (determined from controls).
Model 1 adjusted for maternal age, season of conception, gravidity, parity and maternal education.
Model 2 adjusted for covariates in model 1 plus nitrogen dioxide exposure levels during the same period.
Model 3 adjusted for covariates in model 1 plus particulate matter with an aerodynamic diameter ≤10 μm exposure levels during the same period.
Model 4 adjusted for covariates in model 1 plus nitrogen dioxide and particulate matter with an aerodynamic diameter ≤10 μm exposure levels during the same period.
In the sensitivity analyses, a more balanced subsample of 767 congenital ear malformations cases and 767 matched controls were generated after 1:1 PSM. As shown in Supplementary Table S3, PSM removed the enormous imbalance in selected covariate distributions. SO2 exposure treated as a categorical variable or as a continuous one was strongly associated with the risk of congenital ear malformations during the entire exposure window (Supplementary Table S4).
The results of RCS models are shown in Figure 2. The risks of congenital ear malformations versus SO2 exposure were analyzed by incorporating the selected covariate using the RCS model analyses. And the associations between SO2 exposure and congenital ear malformations risk were further confirmed with non-linear dose-response association during the entire exposure window.
FIGURE 2
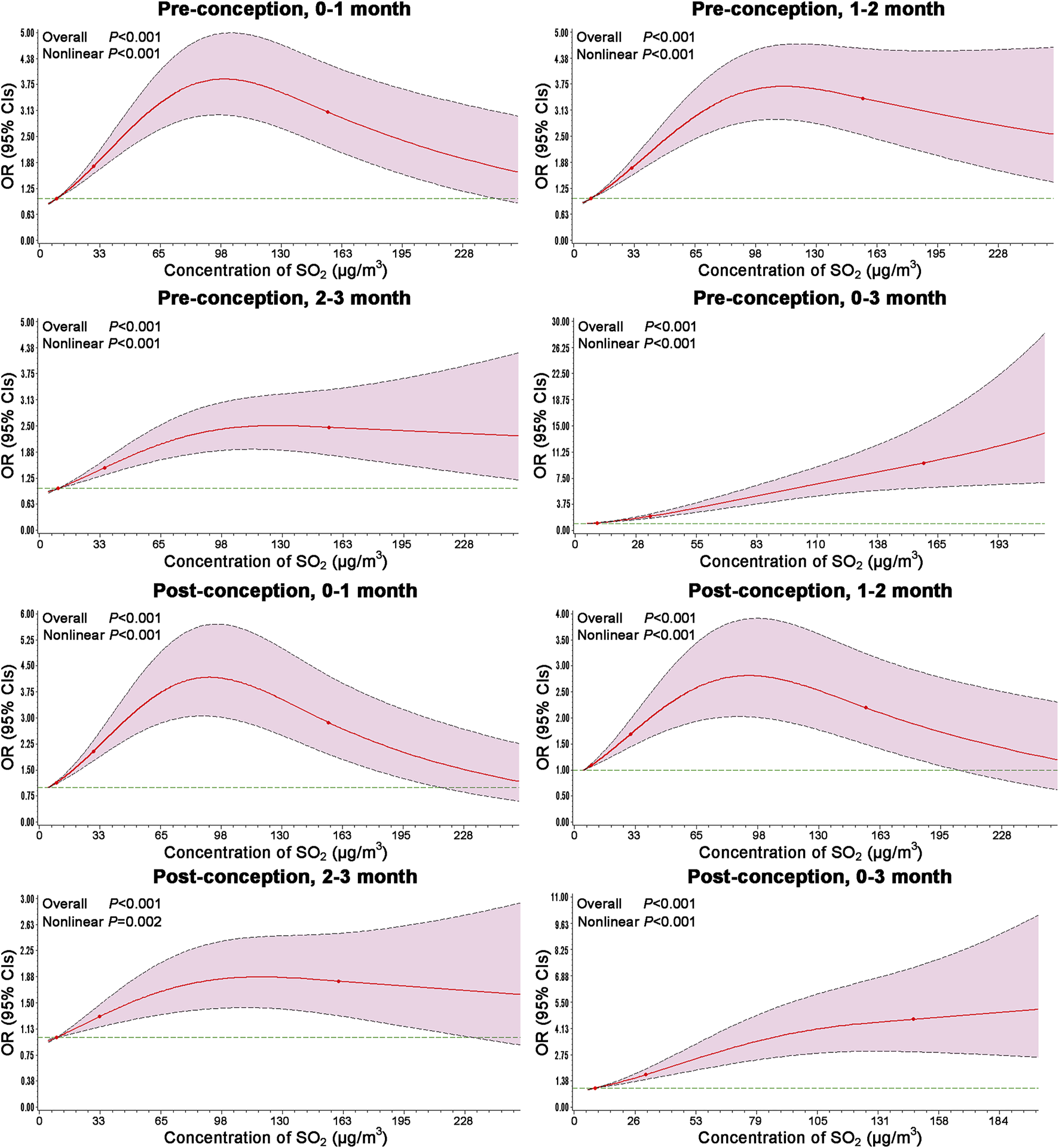
Dose-response associations between ambient SO2 exposure and the risk of congenital ear malformations modeled by RCS. Models adjusted for maternal age, season of conception, gravidity, parity, maternal education, NO2 and PM10 exposure levels during the same period. Odds ratios represented by bold line, and 95% confidence intervals represented by shaded area (Association between maternal exposure to SO2 and congenital ear malformations in offspring: a population-based case-control study in Liaoning Province, China, 2010–2015).
Discussion
This is the first study to examine the association between maternal SO2 exposure and congenital ear malformations risk in offspring. Specifically, we observed significant positive associations during the 3 months before conception and the 3 months after conception. And above associations between SO2 exposure and congenital ear malformations risk were curvilinear and also remained robust after 1:1 PSM. Moreover, subgroups analyses stratified by ear malformation subtype and maternal age were broadly consistent with the main results.
The exact bio-mechanism by which SO2 exposure during conception increases the risk of congenital ear malformations in offspring is still unclear. The auricle develops from the first and the second branchial arches during the embryonic period of 5–9 weeks [28], so it is generally considered to be the joint site of action for the pathological occurrence of congenital microtia [1]. The influencing mechanism behind various risk factors, including air pollution, may be the abnormal migration of cranial neural crest cells that leads to the occurrence of congenital microtia [29]. Evidence from candidate gene and epigenome-wide association studies have suggested that maternal exposure to air pollutants during conception can cause locus-specific changes in methylation, newborn cord blood, and the placenta, particularly in genes involved in cellular responses to oxidative stress, mitochondrial function, inflammation, growth, and early life development [14]. Meanwhile, limited animal experimental studies confirm the association. Research published in 2017 by Calderón-Garcidueñas et al. [16] showed that healthy young dogs in Mexico City exposed to super standard fine particulate matter (PM) and O3 showed an association between auditory nuclei dysmorphology and the brainstem auditory evoked potential (BAEP). It is important to note that an epidemiological study they previously conducted in Mexico City in 2011 had estimated this association, as they were conducting this animal experimental study at the time [30]. Given that zebrafish are morphologically similar to hair cells in the human inner ear, zebrafish are widely used to assess ototoxicity. In their most recent study, Rhee et al. [17] used the zebrafish model to assess damage and developmental toxicity of hair cells caused by PM2.5 exposure. They observed significant hair cell damage after exposure to PM2.5, which was dose-dependent and more severe after prolonged exposure. In vivo experiments conducted by Yadav et al. [18] showed that in the presence of urban particles, the number of Streptococcus pneumoniae in the nasopharynx of mice increased and spread to the middle ear and lungs, thereby causing pathological changes.
The literature on the association between maternal air pollution exposure and congenital ear malformations risk is sparse. To date, only four studies have focused on this research area [31–34], and they are not directly comparable to the results of this study. Although these studies involved different regions, races, study types, sample sizes, covariate controls, exposure assessment, and statistical analysis methods, they have generated consistent findings in that maternal exposure to air pollution increased the risk of congenital ear malformations. The earliest epidemiological study was a population-based case-control study conducted by Rankin et al. [31] in a northern part of the United Kingdom. They found that eye, ear, face, and neck anomalies were positively non-significantly associated with maternal SO2 exposure. Nevertheless, for ear malformations, they failed to provide specific results. Additionally, Pedersen et al. [32] reported that a 10 μg/m3 increment in the NO2 concentration during the first trimester was positively associated with the risk of ear, face, and neck anomalies after adjusting for parental age, maternal smoking, maternal alcohol consumption, maternal education, and disposable income based on the Danish National Birth Cohort. In 2013, Vinikoor-Imler et al. [33] compiled a state-wide birth cohort from North Carolina, which used a hierarchical Bayesian model to assess maternal fine particulate matter (PM2.5) and ozone (O3) exposure levels from weeks 3–8 of gestation. Binomial regression model analysis showed that microtia and anotia were positively correlated with maternal PM2.5 and O3 exposure, but the 95% CI was wide and included the null. Increased odds of microtia and anotia in association with the NO2 concentration have been reported in another study conducted in the United States [34]. Previously, the team had investigated the association between traffic-related air pollution and selected congenital anomalies such as neural tube defects, orofacial clefts, gastroschisis [35], and congenital heart defects [36]. Consistent with the results of Rankin et al. [31], we detected congenital malformations through active surveillance across more than 6 years in a relatively large geographic area and analyzed maternal SO2 exposure as quartiles using the distribution among the compared population. However, we had a relatively larger sample size and higher SO2 exposure level. Furthermore, we adjusted for more covariates. In addition, Pedersen et al. [32] and Padula et al. [34] examined the effects of NO2, but neither reported the risk estimates based on the co-pollutant adjusted model. Interestingly, in our study, after adjustment for NO2, we observed a significant association between maternal SO2 exposure and congenital ear malformations risk, suggesting that this association may be significantly influenced by NO2. However, although data for other air pollutants, such as PM2.5 and O3, may be important for exposure assessment during conception, these data were not available in this study.
Pregnancy is a very critical period that is sensitive to external toxic and harmful substances, including air pollutants. For the attenuating or the masking of association, there is a plausible explanation that the timing of environmental influences for the development of certain congenital malformations is narrow and precise. Congenital malformations mainly occur during the organ-forming period of 3–8 weeks in the first trimester, so the precise window of exposure is crucial in terms of the type of malformations that causes them. For example, the window of ear development is generally 5–8 weeks [37]. The exposure window periods of these four epidemiological studies were different from each other. One study [34] investigated this research topic from the first to the second month of pregnancy, whereas the other [33] focused on the exposure window from weeks 3–8 of pregnancy. Owing to the paucity of epidemiological evidence, it is difficult to arrive at a conclusion at this stage, and further studies are urgently needed.
Strengths and Limitations
One of the strengths of this study is that it provides original findings on the effects of exposure to ambient SO2 during the 3 months before conception and the 3 months after conception and that the SO2 concentration is associated with congenital ear malformations risk. The large sample was selected from the Maternal and Child Health Certificate Registry of Liaoning Province from 2010 to 2015, and it included 1676 cases of congenital ear malformations and 7950 controls, which allowed us to investigate the topic. Additionally, maternal SO2 exposure (data from the Environment Protection Bureau of 14 cities in Liaoning Province) was assessed in detail. The exposure window included the 3 months before conception and the 3 months after conception to ensure that the strength of the association was fully assessed. Finally, we performed sensitivity and subgroup analyses stratified by congenital ear malformations subtypes and maternal age (<30, ≥30 years), and the results showed stability of associations. The RCS combines quantitative data with the strength of association for the occurrence of outcome, enabling a continuous presentation of the association strength dose-response relationship.
However, there are some limitations, and we suggest interpreting our results with caution. Firstly, the assessment of individual SO2 exposure concentrations may have led to exposure misclassification. Because it is difficult to accurately collect individual exposure levels, we used fixed-site monitoring to represent the exposure to pollutants, which inevitably led to bias in exposure assessments. Therefore, to reduce the misclassification of exposure in future studies, it may be necessary to use more sophisticated models to evaluate SO2 exposure such as land-use regression models or spatial interpolation models [38]. Secondly, exposure assessment was based on fixed residential addresses, but the residential address of mothers may have changed during conception. For example, the mobility rate was 9% in the northern UK study [39], but it was 3.1% in the Lanzhou study in China [9]. Nevertheless, Lupo et al. [40] have reported that changes in residential addresses during pregnancy had little effect on these assessments. Thirdly, outdoor air pollutant exposure was only captured, but indoor microenvironment air pollutant exposure, such as homes and workplaces, was not, which may have also led to the misclassification of exposure, and the overestimation or underestimation of effects. Fourthly, although the case information of congenital malformations was obtained through the monitoring network and had various quality controls, it was difficult to guarantee absolute consistency in the process of determining and collecting cases due to the differences in diagnosis among different hospitals, and the longest diagnosis time was 7 days after birth, which may have led to the loss of cases. Finally, other unmeasured covariates, such as maternal disease, maternal or paternal smoking, family history of genetic disease, and toxicity exposure during pregnancy, which have been reported in previous studies as possible risk factors for congenital ear malformations [5, 41], may have influenced our results.
Conclusion
The present study suggests that maternal SO2 exposure is associated with increased risks of congenital ear malformations in offspring. Future studies are still needed to confirm or refute these associations and provide scientific evidence for possible public health intervention during the crucial period of ear development.
Statements
Data availability statement
Detailed analytical data are stored by the authors and are available on request.
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional Review Board of Liaoning Women and Children’s Health Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
W-JY, NL, T-TG, Y-HZ, Y-HH, and Q-JW: conceptualization, methodology, software, writing—original draft, writing—review and editing, and funding acquisition. J-YZ, Y-TJ, JL, SL, Y-LC, L-LL, C-ZJ, and Z-JC: retrieve literature and data curation. W-JY, NL, T-TG, J-YZ, and Y-TJ: methodology, software, writing—review and editing, and validation. Q-JW: supervision and funding acquisition.
Funding
The authors declare that this study was supported by the grants from National Key R&D Program of China (No. 2017YFC0907403), Liaoning Providence science and technology project (No. 2015225025), the Shenyang science and technology project (No. F15-139-9-09), the LiaoNing Revitalization Talents Program (No. XLYC1907102), and 345 Talent Project to T-TG and Q-JW. The funder had the following involvement with the study: conceptualization, methodology, software, writing—original draft, writing—review and editing, supervision, and funding acquisition.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.ssph-journal.org/articles/10.3389/ijph.2022.1604945/full#supplementary-material
Abbreviations
CIs, confidence intervals; NO2, nitrogen dioxide; ORs, odds ratios; O3, ozone; PM, particulate matter; PM10, particulate matter with aerodynamic diameter ≤10 μm; PM2.5, particulate matter with aerodynamic diameters ≤2.5 μm; PSM, propensity score matching; RCS, restricted cubic splines; SD, standard deviation; SO2, sulfur dioxide; Tile, percentile.
References
1.
Hartzell LD Chinnadurai S . Microtia and Related Facial Anomalies. Clin Perinatology (2018) 45(4):679–97. 10.1016/j.clp.2018.07.007
2.
Suutarla S Rautio J Ritvanen A Ala-Mello S Jero J Klockars T . Microtia in Finland: Comparison of Characteristics in Different Populations. Int J Pediatr Otorhinolaryngol (2007) 71(8):1211–7. 10.1016/j.ijporl.2007.04.020
3.
Ministry of Health of China. Ministry of Health of China Publishing Maternal and Child Health Monitoring Web. Beijing, China: Ministry of Health of China (2020).
4.
Chen X Zhang R . Microtia Epigenetics. Medicine (Baltimore) (2019) 98(41):e17468. 10.1097/MD.0000000000017468
5.
Zhang Q-g. Zhang J Yu P Shen H . Environmental and Genetic Factors Associated with Congenital Microtia: a Case-Control Study in Jiangsu, China, 2004 to 2007. Plast Reconstr Surg (2009) 124(4):1157–64. 10.1097/PRS.0b013e3181b454d8
6.
Ostro B Spadaro JV Gumy S Mudu P Awe Y Forastiere F et al Assessing the Recent Estimates of the Global burden of Disease for Ambient Air Pollution: Methodological Changes and Implications for Low- and Middle-Income Countries. Environ Res (2018) 166:713–25. 10.1016/j.envres.2018.03.001
7.
Ritz B Yu F Fruin S Chapa G Shaw GM Harris JA . Ambient Air Pollution and Risk of Birth Defects in Southern California. Am J Epidemiol (2002) 155(1):17–25. 10.1093/aje/155.1.17
8.
Zhang Q Sun S Sui X Ding L Yang M Li C et al Associations between Weekly Air Pollution Exposure and Congenital Heart Disease. Sci Total Environ (2021) 757:143821. 10.1016/j.scitotenv.2020.143821
9.
Jin L Qiu J Zhang Y Qiu W He X Wang Y et al Ambient Air Pollution and Congenital Heart Defects in Lanzhou, China. Environ Res Lett (2015) 10(7):074005. 10.1088/1748-9326/10/7/074005
10.
Ravindra K Chanana N Mor S . Exposure to Air Pollutants and Risk of Congenital Anomalies: A Systematic Review and Metaanalysis. Sci Total Environ (2021) 765:142772. 10.1016/j.scitotenv.2020.142772
11.
Zhu Y Zhang C Liu D Grantz KL Wallace M Mendola P . Maternal Ambient Air Pollution Exposure Preconception and during Early Gestation and Offspring Congenital Orofacial Defects. Environ Res (2015) 140:714–20. 10.1016/j.envres.2015.06.002
12.
Vinceti M Malagoli C Malavolti M Cherubini A Maffeis G Rodolfi R et al Does Maternal Exposure to Benzene and PM 10 during Pregnancy Increase the Risk of Congenital Anomalies? A Population-Based Case-Control Study. Sci Total Environ (2016) 541:444–50. 10.1016/j.scitotenv.2015.09.051
13.
Gianicolo EAL Mangia C Cervino M Bruni A Andreassi MG Latini G . Congenital Anomalies Among Live Births in a High Environmental Risk Area-A Case-Control Study in Brindisi (Southern Italy). Environ Res (2014) 128:9–14. 10.1016/j.envres.2013.11.002
14.
Isaevska E Moccia C Asta F Cibella F Gagliardi L Ronfani L et al Exposure to Ambient Air Pollution in the First 1000 Days of Life and Alterations in the DNA Methylome and Telomere Length in Children: A Systematic Review. Environ Res (2021) 193:110504. 10.1016/j.envres.2020.110504
15.
Slama R Darrow L Parker J Woodruff TJ Strickland M Nieuwenhuijsen M et al Meeting Report: Atmospheric Pollution and Human Reproduction. Environ Health Perspect (2008) 116(6):791–8. 10.1289/ehp.11074
16.
Calderón-Garcidueñas L González-González LO Kulesza RJ Fech TM Pérez-Guillé G Luna MAJ-B et al Exposures to fine Particulate Matter (PM2.5) and Ozone above USA Standards Are Associated with Auditory Brainstem Dysmorphology and Abnormal Auditory Brainstem Evoked Potentials in Healthy Young Dogs. Environ Res (2017) 158:324–32. 10.1016/j.envres.2017.06.026
17.
Rhee J Han E Nam KJ Lim KH Chan Rah Y Park S et al Assessment of Hair Cell Damage and Developmental Toxicity after fine Particulate Matter 2.5 μm (PM 2.5) Exposure Using Zebrafish (Danio rerio) Models. Int J Pediatr Otorhinolaryngol (2019) 126:109611. 10.1016/j.ijporl.2019.109611
18.
Yadav MK Go YY Jun I Chae S-W Song J-J . Urban Particles Elevated Streptococcus Pneumoniae Biofilms, Colonization of the Human Middle Ear Epithelial Cells, Mouse Nasopharynx and Transit to the Middle Ear and Lungs. Sci Rep (2020) 10(1):5969. 10.1038/s41598-020-62846-7
19.
Song C Wu L Xie Y He J Chen X Wang T et al Air Pollution in China: Status and Spatiotemporal Variations. Environ Pollut (2017) 227:334–47. 10.1016/j.envpol.2017.04.075
20.
Zhang J-Y Gong T-T Huang Y-H Li J Liu S Chen Y-L et al Association between Maternal Exposure to PM10 and Polydactyly and Syndactyly: A Population-Based Case-Control Study in Liaoning Province, China. Environ Res (2020) 187:109643. 10.1016/j.envres.2020.109643
21.
Gong T-T Wu Q-J Chen Y-L Jiang C-Z Li J Li L-L et al Evaluating the Time Trends in Prevalence of Exomphalos in 14 Cities of Liaoning Province, 2006 to 2015. Sci Rep (2016) 6:32901. 10.1038/srep32901
22.
Huang Y-H Wu Q-J Chen Y-L Jiang C-Z Gong T-T Li J et al Trends in the Prevalence of Congenital Hydrocephalus in 14 Cities in Liaoning Province, China from 2006 to 2015 in a Population-Based Birth Defect Registry from the Liaoning Women and Children's Health Hospital. Oncotarget (2018) 9(18):14472–80. 10.18632/oncotarget.24239
23.
Chen YL Li CH Huang YH . Incidence of Birth Defects in Liaoning Province, 2006-2015. Chin J Public Health (2018) 34:1662–4.
24.
Xu L Li X Dai L Yuan X Liang J Zhou G et al Assessing the Trend of Gastroschisis Prevalence in China from 1996 to 2007 Using Two Analytical Methods. Birth Defects Res A: Clin Mol Teratology (2011) 91(3):177–84. 10.1002/bdra.20753
25.
Greenland S . Modeling and Variable Selection in Epidemiologic Analysis. Am J Public Health (1989) 79(3):340–9. 10.2105/ajph.79.3.340
26.
Austin PC . Balance Diagnostics for Comparing the Distribution of Baseline Covariates between Treatment Groups in Propensity-Score Matched Samples. Statist Med (2009) 28(25):3083–107. 10.1002/sim.3697
27.
Desquilbet L Mariotti F . Dose-response Analyses Using Restricted Cubic Spline Functions in Public Health Research. Statist Med (2010) 29(9):a–n. 10.1002/sim.3841
28.
Joukhadar N McKee D Caouette-Laberge L Bezuhly M . Management of Congenital Auricular Anomalies. Plast Reconstr Surg (2020) 146(2):205e–216e. 10.1097/PRS.0000000000006997
29.
Watt FM Hogan a. BLM . Out of Eden: Stem Cells and Their Niches. Science (2000) 287(5457):1427–30. 10.1126/science.287.5457.1427
30.
Calderón‐Garcidueñas L D'Angiulli A Kulesza RJ Torres‐Jardón R Osnaya N Romero L et al Air Pollution Is Associated with Brainstem Auditory Nuclei Pathology and Delayed Brainstem Auditory Evoked Potentials. Int J Dev Neurosci (2011) 29(4):365–75. 10.1016/j.ijdevneu.2011.03.007
31.
Rankin J Chadwick T Natarajan M Howel D Pearce MS Pless-Mulloli T . Maternal Exposure to Ambient Air Pollutants and Risk of Congenital Anomalies. Environ Res (2009) 109(2):181–7. 10.1016/j.envres.2008.11.007
32.
Pedersen M Garne E Hansen-Nord N Hjortebjerg D Ketzel M Raaschou-Nielsen O et al Exposure to Air Pollution and Noise from Road Traffic and Risk of Congenital Anomalies in the Danish National Birth Cohort. Environ Res (2017) 159:39–45. 10.1016/j.envres.2017.07.031
33.
Vinikoor-Imler LC Davis JA Meyer RE Luben TJ . Early Prenatal Exposure to Air Pollution and its Associations with Birth Defects in a State-wide Birth Cohort from North Carolina. Birth Defects Res Part A: Clin Mol Teratology (2013) 97(10):696–701. 10.1002/bdra.23159
34.
Padula AM Tager IB Carmichael SL Hammond SK Yang W Lurmann FW et al Traffic-related Air Pollution and Selected Birth Defects in the San Joaquin Valley of California. Birth Defects Res Part A: Clin Mol Teratology (2013) 97(11):730–5. 10.1002/bdra.23175
35.
Padula AM Tager IB Carmichael SL Hammond SK Lurmann F Shaw GM . The Association of Ambient Air Pollution and Traffic Exposures with Selected Congenital Anomalies in the San Joaquin Valley of California. Am J Epidemiol (2013) 177(10):1074–85. 10.1093/aje/kws367
36.
Padula AM Tager IB Carmichael SL Hammond SK Yang W Lurmann F et al Ambient Air Pollution and Traffic Exposures and Congenital Heart Defects in the San Joaquin Valley of California. Paediatr Perinat Epidemiol (2013) 27(4):329–39. 10.1111/ppe.12055
37.
Klockars T Rautio J . Embryology and Epidemiology of Microtia. Facial Plast Surg (2009) 25(3):145–8. 10.1055/s-0029-1239444
38.
Saenen ND Vrijens K Janssen BG Roels HA Neven KY Vanden Berghe W et al Lower Placental Leptin Promoter Methylation in Association with Fine Particulate Matter Air Pollution during Pregnancy and Placental Nitrosative Stress at Birth in the ENVIR ON AGE Cohort. Environ Health Perspect (2017) 125(2):262–8. 10.1289/EHP38
39.
Hodgson S Shirley M Bythell M Rankin J . Residential Mobility during Pregnancy in the north of England. BMC Pregnancy Childbirth (2009) 9:52. 10.1186/1471-2393-9-52
40.
Lupo PJ Symanski E Chan W Mitchell LE Waller DK Canfield MA et al Differences in Exposure Assignment between conception and Delivery: the Impact of Maternal Mobility. Paediatr Perinat Epidemiol (2010) 24(2):200–8. 10.1111/j.1365-3016.2010.01096.x
41.
Mastroiacovo P Corchia C Botto LD Lanni R Zampino G Fusco D . Epidemiology and Genetics of Microtia-Anotia: a Registry Based Study on over One Million Births. J Med Genet (1995) 32(6):453–7. 10.1136/jmg.32.6.453
Summary
Keywords
air pollution, congenital ear malformations, microtia, risk, sulfur dioxide
Citation
Yu W-J, Li N, Gong T-T, Zhang J-Y, Jiang Y-T, Zhao Y-H, Huang Y-H, Li J, Liu S, Chen Y-L, Li L-L, Jiang C-Z, Chen Z-J and Wu Q-J (2022) Association Between Maternal Exposure to SO2 and Congenital Ear Malformations in Offspring: A Population-Based Case-Control Study in Liaoning Province, China. Int J Public Health 67:1604945. doi: 10.3389/ijph.2022.1604945
Received
24 March 2022
Accepted
09 June 2022
Published
07 July 2022
Volume
67 - 2022
Edited by
Nino Kuenzli, Swiss Tropical and Public Health Institute (Swiss TPH), Switzerland
Reviewed by
Sanghyuk Bae, Catholic University of Korea, South Korea
Emilio Gianicolo, Johannes Gutenberg University Mainz, Germany
Updates
Copyright
© 2022 Yu, Li, Gong, Zhang, Jiang, Zhao, Huang, Li, Liu, Chen, Li, Jiang, Chen and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting-Ting Gong, gongtt@sj-hospital.org; Qi-Jun Wu, wuqj@sj-hospital.org
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.